Amitriptyline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- Suicidality and Antidepressant Drugs
- AMITRIPTYLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- AMITRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- AMITRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- METABOLISM
- REFERENCES
- MEDICATION GUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
Suicidality and Antidepressant Drugs
WARNINGS: Clinical Worsening and Suicide Risk, PRECAUTIONS: Information for Patients, and PRECAUTIONS: Pediatric Use)AMITRIPTYLINE HYDROCHLORIDE DESCRIPTION
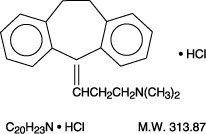
10 mg also includes D&C Red #27 Aluminum Lake, D&C Yellow #10 Aluminum Lake and FD&C Blue #1 Aluminum Lake;25 mg- D&C Yellow #10 Aluminum Lake, FD&C Blue #1 Aluminum Lake and FD&C Red #40 Aluminum Lake;50 mg-FD&C Blue #2 Aluminum Lake and FD&C Red #40 Aluminum Lake;75 mg-D&C Red #7 Calcium Lake and FD&C Blue #2 Aluminum Lake;100 mg-D&C Red #30 Aluminum Lake and D&C Yellow #10 Aluminum Lake;150 mg-D&C Yellow #10 Aluminum Lake, FD&C Blue #1 Aluminum Lake and FD&C Red #40 Aluminum Lake.
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
AMITRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Table 1
Age Range Drug-Placebo Difference in
Number of Cases of Suicidality
per 1000 Patients Treated
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.Prescriptions for amitriptyline hydrochloride should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believe (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that amitriptyline hydrochloride is not approved for use in treating bipolar depression.
Usage in Pregnancy
1). Studies in literature have shown amitriptyline to be teratogenic in mice and hamsters when given by various routes of administration at doses of 28 to 100 mg/kg/day (9 to 33 times the maximum recommended human dose), producing multiple malformations. Another study in the rat reported that an oral dose of 25 mg/kg/day (8 times the maximum recommended human dose) produced delays in ossification of fetal vertebral bodies without other signs of embryotoxicity. In rabbits, an oral dose of 60 mg/kg/day (20 times the maximum recommended human dose) was reported to cause incomplete ossification of cranial bones.
1
Nursing Mothers
Usage in Pediatric Patients
PRECAUTIONS
Information for Patients
Clinical Worsening and Suicide Risk
Drug Interactions
CONTRAINDICATIONSseeWARNINGSsection.
Geriatric Use
DOSAGE AND ADMINISTRATION).
Pediatric Use
BOX WARNINGandWARNINGS: Clinical Worsening and Suicide Risk). Anyone considering the use of amitriptyline in a child or adolescent must balance the potential risks with the clinical need.
AMITRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
Cardiovascular:Myocardial infarction; stroke; nonspecific ECG changes and changes in AV conduction; heart block; arhythmias; hypotension, particularly orthostatic hypotension; syncope; hypertension; tachycardia; palpitation.
CNS and Neuromuscular:Coma; seizures; hallucinations; delusions; confusional states; diorientation; incoordination; ataxia; tremors; peripheral neuropathy; numbness, tingling and paresthesias of the extremities; extrapyramidal symptoms including abnormal involuntary movements and tardive dyskinesia; dysarthria; disturbed concentration; excitement; anxiety; insomnia; restlessness; nightmares; drowsiness; dizziness; weaknessl fatigue; headache; syndrome of inappropriate ADH (antidiuretic hormone) secretion; tinnitus; alteration in EEG patterns.
Anticholinergic:Paralytic ileus, hyperpyrexia; urinary retention, dilatation of the urinary tract; constipation; blurred vision, disturbance of accommodation, increased ocular pressure, mydriasis; dry mouth.
Allergic:Skin rash; urticaria; photosensitization; edema of face and tongue.
Hematologic:Bone marrow depression including agranulocytosis, leukopenia, thrombocytopenia; purpura; eosinophilia.
Gastrointestinal:Rarely hepatitis (including altered liver function and jaundice); nausea; epigastric distress; vomiting; anorexia; stomatitis; peculiar taste; diarrhea; parotid swelling; black tongue.
Endocrine:Testicular swelling and gynecomastia in the male; breast enlargement and galactorrhea in the female; increased or decreased libido; impotence; elevation and lowering of blood sugar levels.
Other:Alopecia; edema; weight gain or loss; urinary frequency; increased perspiration.
Withdrawal Symptoms
Causal Relationship Unknown
Body as a Whole:Lupus-like syndrome (migratory arthritis, positive ANA and rheumatoid factor).
Digestive:Hepatic failure, ageusia.
Postmarketing Adverse Events
OVERDOSAGE
Manifestations
ADVERSE REACTIONS.
Management
Psychiatric Follow-up
Pediatric Management
DOSAGE & ADMINISTRATION
Oral DosageInitial Dosage for Adults
Adolescent and Elderly Patients
Maintenance
Usage in Pediatric Patients
Plasma Levels
2
2 Hollister, L.E.; Monitoring Tricyclic Antidepressant Plasma Concentrations. JAMA 1979; 241(23):2530-2533.
HOW SUPPLIED
10 mg:Round, film-coated pink tablets, debossed GG 40 on one side and plain on the reverse side, and supplied as:
25 mg:Round, film-coated light green tablets, debossed GG 44 on one side and plain on the reverse side, and supplied as:
50 mg:Round, film-coated brown tablets, debossed GG 431 on one side and plain on the reverse side, and supplied as:
75 mg:Round, film-coated purple tablets, debossed GG 451 on one side and plain on the reverse side, and supplied as:
100 mg:Round, film-coated orange tablets, debossed GG 461 on one side and plain on the reverse side, and supplied as:
150 mg:Capsule shaped, film-coated light green tablets, debossed GG 450 on one side and plain on the reverse side, and supplied as:
STORAGE AND HANDLING
METABOLISM
REFERENCES
MEDICATION GUIDE
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or ActionsTalk to your, or your family memberhealthcare provider about:
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
What else do I need to know about antidepressant medicines?
Never stop an antidepressant medicine without first talking to a healthcare provider.Stopping an antidepressant medicine suddenly can cause other symptoms.
Antidepressants are medicines used to treat depression and other illnesses.It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
Antidepressant medicines have other side effects.Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
Antidepressant medicines can interact with other medicines.Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
Not all antidepressant medicines prescribed for children are FDA approved for use in children.Talk to your childhealthcare provider for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Amitriptyline HydrochlorideAMITRIPTYLINE HYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!