Amiodarone Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- AMIODARONE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- AMIODARONE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- AMIODARONE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
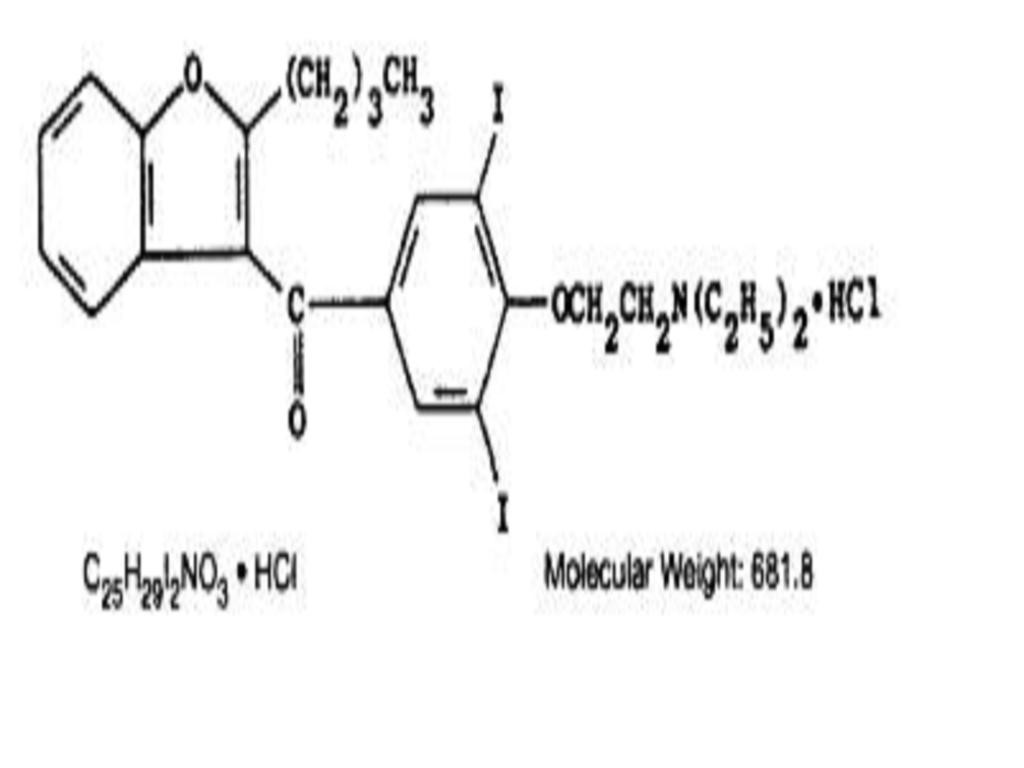
AMIODARONE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
Electrophysiology/Mechanisms of Action:WARNINGS
Hemodynamics:
Pharmacokinetics:
DOSAGE AND ADMINISTRATION
Pharmacodynamics:
Monitoring Effectiveness:
INDICATIONS & USAGE
WARNINGSAMIODARONE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Mortality:
Pulmonary Toxicity:
Worsened Arrhythmia:
Other reported interactions with amiodarone
Thyrotoxicosis:
Thyroid Abnormalities
Liver Injury:
Loss of Vision:
ADVERSE REACTIONS
Neonatal Hypo- or Hyperthyroidism:
PRECAUTIONS
Impairment of Vision:Optic Neuropathy and/or Neuritis:
WARNINGS
Corneal Microdeposits:
ADVERSE REACTIONS
Neurologic:
Photosensitivity:
Thyroid Abnormalities:
Thyrotoxicosis
WARNINGSADVERSE REACTIONS
Surgery:
Volatile Anesthetic Agents:
Hypotension Postbypass:
Adult Respiratory Distress Syndrome (ARDS):
Corneal Refractive Laser Surgery:
Information for Patients
Laboratory Tests:
Drug Interactions:
Pharmacokinetics
Protease inhibitors:
Antidepressants:
Other substances:
DOSAGE AND ADMINISTRATION
Immunosuppressives:
HMG-CoA reductase inhibitors:
Cardiovasculars:
Cardiac glycosides:
Antiarrhythmics:
Antihypertensives:
Anticoagulants:
Antibiotics:
Other substances, including herbal preparations:
Other reported interactions with amiodarone:
Worsened Arrhythmia
Volatile Anesthetic Agents:
Volatile Anesthetic Agents
Electrolyte Disturbances:
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category D:Neonatal Hypo- or Hyperthyroidism
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
AMIODARONE HYDROCHLORIDE ADVERSE REACTIONS
WARNINGSPRECAUTIONSWARNINGS
The following side effects were each reported in 10 to 33% of patients:
The following side effects were each reported in 4 to 9% of patients:
The following side effects were each reported in 1 to 3% of patients:
The following side effects were each reported in less than 1% of patients:
Postmarketing Reports:
OVERDOSAGE
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGY
For life-threatening ventricular arrhythmias, such as ventricular fibrillation or hemodynamically unstable ventricular tachycardia:
Drug Interactions
Drug InteractionsMonitoring EffectivenessCLINICAL PHARMACOLOGY
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
What is the most important information I should know about Amiodarone Hydrochloride Tablets?
-
● lung damage
-
● liver damage
-
● worse heartbeat problems
-
● thyroid problems
-
● Call your doctor or get medical help right away if you have any symptoms such as the following:
-
● nausea or vomiting; passing brown or dark-colored urine; feel more tired than usual; your skin and whites of your eyes get yellow; or have stomach pain
-
● heart pounding, skipping a beat, beating very fast or very slowly; feel light-headed or faint
-
● weakness, weight loss or weight gain, heat or cold intolerance, hair thinning, sweating, changes in your menses, swelling of your neck (goiter), nervousness, irritability, restlessness, decreased concentration, depression in the elderly, or tremor.
-
● Because of these possible side effects, amiodarone hydrochloride tablets should only be used in adults with life-threatening heartbeat problems called ventricular arrhythmias, for which other treatments did not work or were not tolerated.
What are Amiodarone Hydrochloride Tablets?
-
● have an allergy to amiodarone, iodine, or any of the other ingredients in amiodarone hydrochloride tablets. See the end of this Medication Guide for a complete list of ingredients in amiodarone hydrochloride tablets.
-
●
-
● have liver problems
-
● have or had thyroid problems
-
● have blood pressure problems
-
● are pregnant or planning to become pregnant. Amiodarone can harm your unborn baby. Amiodarone can stay in your body for months after treatment is stopped. Therefore, talk with your doctor before you plan to get pregnant.
-
● are breastfeeding. Amiodarone passes into your milk and can harm your baby. You should not breast-feed while taking amiodarone. Also, amiodarone can stay in your body for months after treatment is stopped.
-
● Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Amiodarone hydrochloride tablets and certain other medicines can interact with each other causing serious side effects. Sometimes the dose of amiodarone hydrochloride tablets or other medicines must be changed when they are used together. Especially, tell your doctor if you are taking:
-
● depression medicines
-
● blood thinner medicines
-
● HIV or AIDS medicines
-
● cimetidine (Tagameta medicine for stomach ulcers or indigestion
-
● loratadine (for example: ClaritinAlaverta medicine for allergy symptoms
-
● seizure medicines
-
● diabetes medicines
-
● cyclosporine, an immunosuppressive medicine
-
● dextromethorphan, a cough medicine
-
● medicines for your heart, circulation, or blood pressure
-
● water pills (diuretics)
-
● high cholesterol or bile medicines
-
● narcotic pain medicines
-
● St. John's Wort
-
● Know the medicines you take. Keep a list of them with you at all times and show it to your doctor and pharmacist each time you get a new medicine. Do not take any new medicines while you are taking amiodarone hydrochloride tablets unless you have talked with your doctor.
How should I take Amiodarone Hydrochloride Tablets?
-
● The dose of amiodarone hydrochloride tablets you take has been specially chosen for you by your doctor and may change during treatment. Keep taking your medicine until your doctor tells you to stop. Do not stop taking it because you feel better. Your condition may get worse. Talk with your doctor if you have side effects.
-
● Your doctor will tell you to take your dose of amiodarone hydrochloride tablets with or without meals. Make sure you take amiodarone hydrochloride tablets the same way each time.
-
● Do not drink grapefruit juice during treatment with amiodarone hydrochloride tablets. Grapefruit juice affects how amiodarone hydrochloride tablets are absorbed in the stomach.
-
● Taking too many amiodarone hydrochloride tablets can be dangerous. If you take too many amiodarone hydrochloride tablets, call your doctor or go to the nearest hospital right away. You may need medical care right away.
-
● If you miss a dose, do not take a double dose to make up for the dose you missed. Continue with your next regularly scheduled dose.
-
●
-
● Avoid exposing your skin to the sun or sun lamps. Amiodarone hydrochloride tablets can cause a photosensitive reaction. Wear sun-block cream or protective clothing when out in the sun.
-
● Avoid pregnancy during treatment with amiodarone hydrochloride tablets. Amiodarone hydrochloride tablets can harm your unborn baby.
-
● Do not breastfeed while taking amiodarone hydrochloride tablets. Amiodarone passes into your milk and can harm your baby.
-
●
What is the most important information I should know about amiodarone hydrochloride tablets?
-
● nerve problems. Amiodarone hydrochloride tablets can cause a feeling of "pins and needles" or numbness in the hands, legs, or feet, muscle weakness, uncontrolled movements, poor coordination, and trouble walking.
-
● thyroid problems. Amiodarone hydrochloride tablets can cause thyroid problems, including low thyroid function or overactive thyroid function. Your doctor may arrange regular blood tests to check your thyroid function during treatment with amiodarone hydrochloride tablets. Call your doctor if you have weakness, weight loss or weight gain, heat or cold intolerance, hair thinning, sweating, changes in your menses, swelling of your neck (goiter), nervousness, irritability, restlessness, decreased concentration, depression in the elderly, or tremor.
-
● skin problems. Amiodarone hydrochloride tablets can cause your skin to be more sensitive to the sun or to turn a bluish-gray color. In most patients, skin color slowly returns to normal after stopping amiodarone hydrochloride tablets. In some patients, skin color does not return to normal.
-
● Other side effects of amiodarone hydrochloride tablets include nausea, vomiting, constipation, and loss of appetite.
How should I store Amiodarone Hydrochloride Tablets?
-
● Safely dispose of amiodarone hydrochloride tablets that are out-of-date or no longer needed.
-
● Keep amiodarone hydrochloride tablets and all medicines out of the reach of children.
-
●
What are the ingredients in Amiodarone Hydrochloride Tablets?
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Amiodarone HydrochlorideAmiodarone Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!