Amantadine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- AMANTADINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- AMANTADINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- AMANTADINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
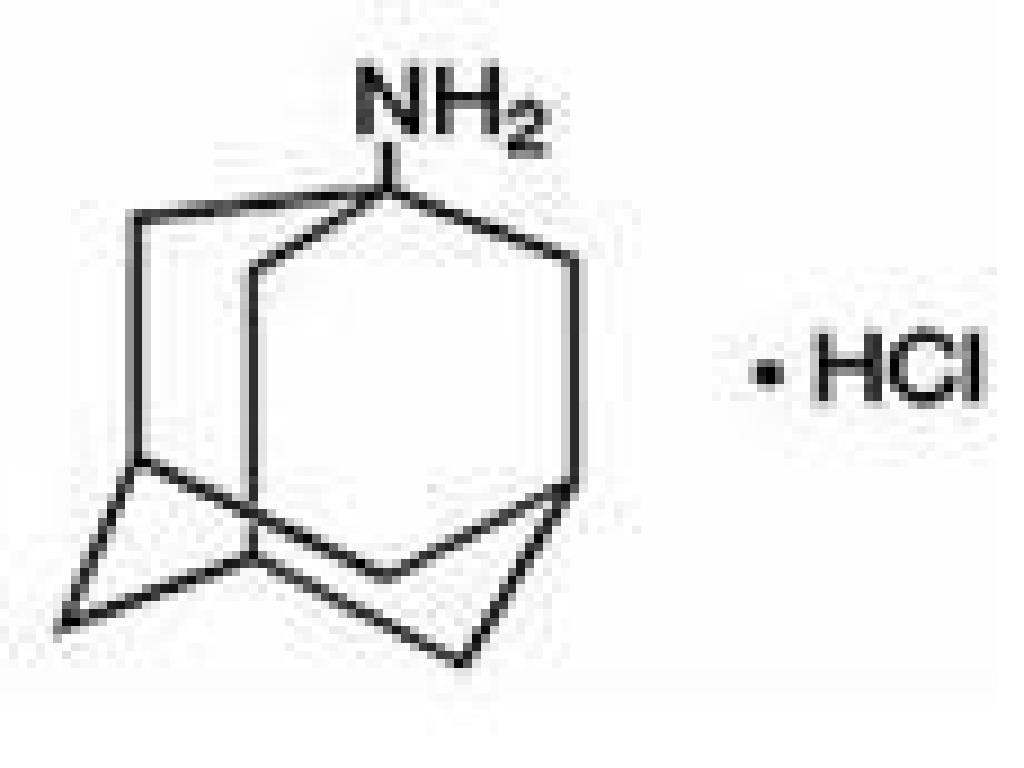
AMANTADINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
Mechanism of Action: AntiviralAntiviral Activity
Drug Resistance
Mechanism of Action: Parkinson's Disease
PHARMACOKINETICS
INDICATIONS & USAGE
Influenza A Prophylaxis
Influenza A Treatment
-
● Amantadine Hydrochloride Capsules, USP is not a substitute for early vaccination on an annual basis as recommended by the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices.
-
● Influenza viruses change over time. Emergence of resistance mutations could decrease drug effectiveness. Other factors (for example, changes in viral virulence) might also diminish clinical benefit of antiviral drugs. Prescribers should consider available information on influenza drug susceptibility patterns and treatment effects when deciding whether to use Amantadine Hydrochloride Capsules, USP.
-
●
Drug-Induced Extrapyramidal Reactions
AMANTADINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
DeathsOVERDOSAGE
Suicide Attempts
CNS Effects
Other
PRECAUTIONS
Neuroleptic Malignant Syndrome (NMS)
Renal disease
Liver disease
Melanoma
Other
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Impairment of Fertility
PREGNANCY
Category CNURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
DOSAGE AND ADMINISTRATIONAMANTADINE HYDROCHLORIDE ADVERSE REACTIONS
WARNINGS
Other adverse reactions reported during postmarketing experience with amantadine usage include:
WARNINGS
Laboratory Test -elevated: CPK, BUN, serum creatinine, alkaline phosphatase, LDH, bilirubin, GGT, SGOT, and SGPT.
OVERDOSAGE
DOSAGE & ADMINISTRATION
Dosage for Impaired Renal FunctionDosage for Prophylaxis and Treatment of Uncomplicated Influenza A Virus Illness
Adult
Pediatric Patients
1 yr.-9 yrs. of age
9 yrs.-12 yrs. of age
Dosage for Parkinsonism
Adult
Dosage for Concomitant Therapy
Dosage for Drug-Induced Extrapyramidal Reactions
Adult
Dosage for Impaired Renal Function
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Amantadine HydrochlorideAmantadine Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!