Alphaquin HP
ALPHAQUIN HP 4% CREAM Rx (HYDROQUINONE 4% USPSKIN BLEACHING MOISTURIZINGTOPICAL CREAM WITH SUNSCREENS)
FULL PRESCRIBING INFORMATION: CONTENTS*
- ALPHAQUIN HP DESCRIPTION
- CLINICAL PHARMACOLOGY
- ALPHAQUIN HP INDICATIONS AND USAGE
- ALPHAQUIN HP CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ALPHAQUIN HP ADVERSE REACTIONS
- OVERDOSAGE
- ALPHAQUIN HP DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- HYDROQUINONE REFERENCES
- PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
FULL PRESCRIBING INFORMATION
Rx only
FOR EXTERNAL USE ONLY: NOT FOR OPHTHALMIC, NASAL OR OTIC USE.
ALPHAQUIN HP DESCRIPTION
Each gram of ALPHAQUIN® HP 4% CREAM Contains: ACTIVE: 40 mg of Hydroquinone USP, 7.5% Octyl Methoxycinnamate,5.0% Benzophenone-3 in a cream base of: INACTIVES: Purified Water, Propylene Glycol, Glycerine, Cetyl Alcohol, Cetostearyl Alcohol, Glycolic Acid, Isopropyl Palmitate, Stearyl Alcohol, Sodium Lauryl Sulfate, Sodium Metabisulfite, Citric Acid, Sorbic Acid and Sodium Hydroxide.
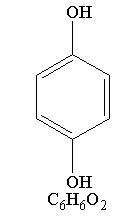
Hydroquinone is 1, 4-Benzenediol, with a chemical formula of C6H6O2 and a molecular weight of 110.11.
The structural formula is:
CLINICAL PHARMACOLOGY
Topical application of hydroquinone produces a reversible depigmentation of the skin by the inhibition of the enzymatic oxidation of tyrosine to 3, 4-dihydroxyphenylalanine (DOPA) [Denton, C., et al., 1952 (1)] and suppression of other melanocyte metabolic processes [Jimbow, K., et al., 1974 (2)]. Exposure to sunlight or ultraviolet light will cause repigmentation of the bleached areas [Parrish, J.A. et al., 1978 (3)].
ALPHAQUIN HP INDICATIONS AND USAGE
ALPHAQUIN® HP 4% CREAM is indicated for the gradual bleaching of hyperpigmented skin conditions such as chloasma, melasma, freckles, senile lentigines and other undesired areas of melanin hyperpigmentation.
ALPHAQUIN HP CONTRAINDICATIONS
ALPHAQUIN® HP 4% CREAM is contraindicated in persons who have shown hypersensitivity to hydroquinone or any of the other ingredients. The safety of topical treatment of hydroquinone during pregnancy or in children (12 years and under) has not been established. (See PRECAUTIONS).
WARNINGS
- Hydroquinone is a skin bleaching agent which may produce undesired effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
- To evaluate possible susceptibility to irritation or sensitivity, each patient should begin by applying the medication to a small portion of unbroken skin at or near the pigmented area over a period of several days. Minor redness is not necessarily a contraindication, but treatment should be discontinued if itching, excessive inflammation or vesicle formation occurs. Close patient supervision is recommended. Use of ALPHAQUIN® HP 4% CREAM in paranasal and infraorbital areas increases the chance of irritations (See ADVERSE REACTIONS). If no improvement is seen after two months of treatment, use of this product should be discontinued.
- Sunscreen use is an essential aspect of hydroquinone therapy since even minimal sunlight exposure stimulates melanocyte activity. The sunscreens in ALPHAQUIN® HP 4% CREAM provide the necessary sun protection during skin bleaching activity. During the depigmentation maintenance treatment subsequent to the intensive depigmentation therapy, sun exposure of the bleached skin should be avoided to prevent repigmentation.
- Contains Sodium Metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
- Keep this and all other medications out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
- Avoid contact with eyes. In case of accidental contact, patient should rinse eyes thoroughly with water and contact physician. A bitter taste and antiseptic effect may occur if applied to the lips.
PRECAUTIONS
(SEE WARNINGS)
Pregnancy Category C
Animal reproduction studies have not been conducted with hydroquinone. It is also not known whether hydroquinone can cause fetal harm when administered on a pregnant woman or can affect reproductive capacity. It is also not known to what degree, if any, topical hydroquinone is absorbed systemically. Topical hydroquinone should be used in pregnant women only when clearly indicated.
Nursing Mothers
It is not known whether topical administration of hydroquinone is absorbed or excreted in human milk. Caution is advised when topical hydroquinone is used by a nursing mother.
Pediatric Use
Safety and effectiveness in children below the age of 12 years has not been established.
ALPHAQUIN HP ADVERSE REACTIONS
No systemic adverse reactions have been reported. Occasional hypersensitivity (localized contact dermatitis) may occur in which case the medication should be discontinued and the physician notified immediately.
OVERDOSAGE
There have been no systemic reactions reported from the use of topical hydroquinone treatment. However, treatment should be limited to relatively small areas of the body at one time since some patients may experience a transient skin reddening and a mild burning sensation which does not preclude treatment.
ALPHAQUIN HP DOSAGE AND ADMINISTRATION
ALPHAQUIN® HP 4% CREAM should be applied to affected areas twice daily, in the morning and before bedtime, or as directed by a physician. Unnecessary solar exposure should be avoided. There is no recommended dosage for children under 12 years of age except under the advice and supervision of a physician. Keep container tightly closed. NOTE: Slight darkening of ALPHAQUIN® HP 4% CREAM is normal and does not affect potency of the product.
HOW SUPPLIED
ALPHAQUIN® HP 4% CREAM (Hydroquinone, USP 4%) is supplied as follows:
| SIZE | NDC NUMBER |
|---|---|
| 1 oz tube (28.4 grams) | 58980-580-10 |
| 2 oz tube (56.7 grams) | 58980-580-20 |
STORAGE
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C and 30°C (between 59°F and 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinectic temperature does not exceed 25°C (77°F); however, such exposure should be minimized. [See USP Controlled Room Temperature]. Protect from freezing. If freezing occurs, warm to room temperature.
Keep out of the reach of children.
Distributed by:
STRATUS
STRATUS PHARMACEUTICALS INC
12379 SW 130TH STREET
MIAMI, FLORIDA 33186
Manufactured by:
Sonar Products, Inc.
Carlstadt, NJ 07072
Copyright ©1993, Stratus Pharmaceuticals, Inc.
All rights reserved.
HYDROQUINONE REFERENCES
- Denton, C., Lerner, A., and Fitzpatrick, T., "Inhibition of Melanin Formation by Chemical Agents", J. Invest. Derm., 18:119-135, 1952.
- Jimbow, K., Obata, M., Pathak, M., and Fitzpatrick, T., "Mechanisms of Depigmentation by Hydroquinone" J. Invest. Derm., 62:436-449, 1974.
- Parrish, J, Anderson, R., Urbach, F., and Pitts, D., " Biological Effects of Ultraviolet Radiation with Emphasis on Human Responses to Longwave Ultraviolet", Plenum Press, New York, 1978 (page 151).
Rev. JG-ACI 2012-304
PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
NDC 58980-580-10
Net WT. 1 OZ (28.4g)
ALPHAQUIN® HP 4% CREAM
STRATUS
PHARMACEUTICALS INC
(HYDROQUINONE USP, 4%)
SKIN BLEACHING CREAM WITH SUNSCREENS
Rx only
Distributed by Stratus Pharmaceuticals Inc., 12379 SW 130th Street, Miami, Florida 33186

Alphaquin HPHydroquinone, Octinoxate, and Oxybenzone CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||