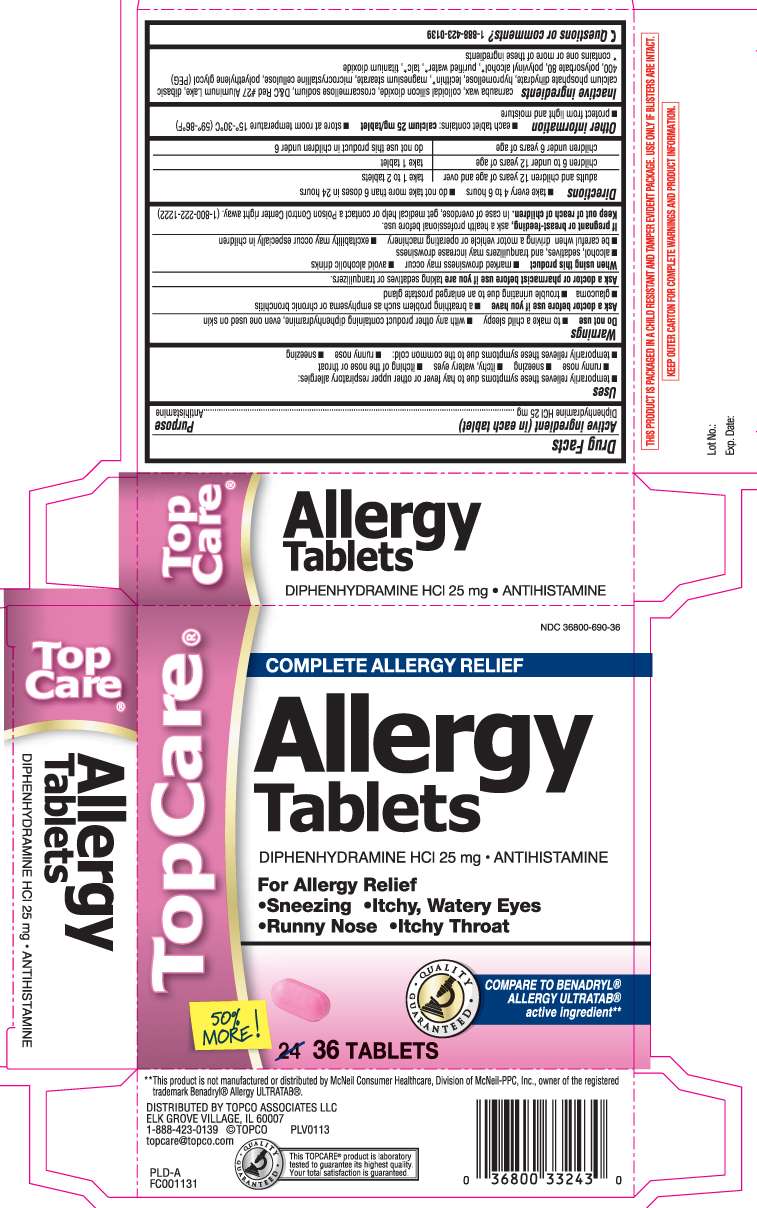
Allergy Relief
TOP CARE (Topco Associates LLC)
P and L Development of New York Corporation
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- Allergy Relief Uses
- Warnings
- Directions
- Allergy Relief Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
- Product Label
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Diphenhydramine HCl 25 mg
Purpose
Antihistamine
Allergy Relief Uses
- temporarily relieves symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers.
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur especially in children
If pregnant of breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
| adults and children 12 years of age and over | take 1 to 2 tablets |
| children 6 to under 12 years of age | take 1 tablet |
| children under 6 years of age | do not use this product in children under 6 |
Allergy Relief Other information
- each tablet contains: calcium 25 mg/tablet
- store at room temperature 15°-30°C (59°-86°F)
- protect from light and moisture
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, D&C Red #27 Aluminum Lake, dibasic calcium phosphate dihydrate, hypromellose, lecithin*, magnesium stearate, microcrystalline cellulose, polyethylene glycol (PEG) 400, polysorbate 80, polyvinyl alcohol*, purified water*, talc*, titanium dioxide
* contains one or more of these ingredients
Questions or comments?
1-888-423-0139
Principal Display Panel
COMPLETE ALLERGY RELIEF
Allergy Tablets
DIPHENHYDRAMINE HCl 25 mg • ANTIHISTAMINE
For allergy relief
•Sneezing •Itchy, Watery Eyes •Runny Nose •Itchy Throat
COMPARE TO BENADRYL® ALLERGY ULTRATAB® active ingredient**
TABLETS
**This product is not manufactured or distributed by McNeil Consumer Healthcare , Division of McNeil-PPC, Inc., owner of the registered trademark Benadryl® Allergy ULTRATAB®.
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007
1-888-423-0139 ©TOPCO topcare@topco.com
THIS PRODUCT IS PACKAGED IN A CHILD RESISTANT AND TAMPER EVIDENT PACKAGE, USE ONLY IF BLISTERS ARE INTACT.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Product Label
Allergy ReliefDiphenhydramine Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||