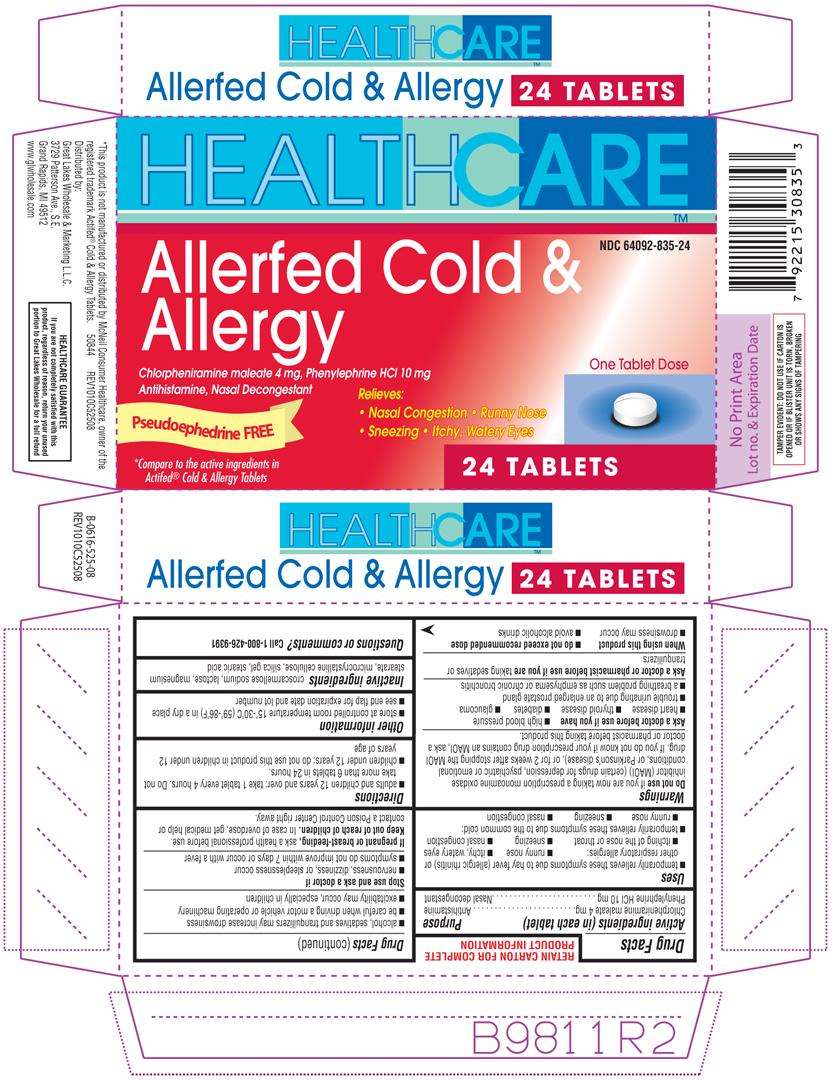
Allerfed Cold and Allergy
GREAT LAKES WHOLESALE, MARKETING, & SALES, INC.
Healthcare 44-525
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients (in each tablet)
- Purpose
- Allerfed Cold and Allergy Uses
- Warnings
- Directions
- Allerfed Cold and Allergy Other information
- Inactive ingredients
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredients (in each tablet)
Chlorpheniramine maleate 4 mg
Phenylephrine HCl 10 mg
Purpose
Antihistamine
Nasal decongestant
Allerfed Cold and Allergy Uses
- temporarily relieves these symptoms due to har fever (allergic rhinitis) or other respiratory allergies:
- runny nose
- itchy, watery eyes
- itching of the nose or throat
- sneezing
- nasal congestion
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
- nasal congestion
Warnings
Do not use
- high blood pressure
- heart disease
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor before use if
- high blood pressure
- heart disease
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
- do not exceed recommended dose
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives and tranquilizers may inscrease drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- symptoms do not improce within 7 days or occur with a fever
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years and over: take 1 tablet every 4 hours. Do not take more than 6 tablets in 24 hours
- children under 12 years: do not use this product in children under 12 years of age
Allerfed Cold and Allergy Other information
- store at controlled room temperature 15º-30ºC (59º-86ºF) in a dry place
- see end flap for expiration date and lot number
Inactive ingredients
croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, silica gel, stearic acid
Principal Display Panel
HEALTHCARE™
NDC 64092-835-24
Allerfed Cold &
Allergy
Chlorpheniramine maleate 4 mg, Phenylephrine HCl 10 mg
Antihistamine, Nasal Decongestant
Pseudoephedrine FREE
Relieves:
• Nasal Congestion • Runny Nose
• Sneezing • Itchy, Watery Eyes
One Tablet Dose
*Compare to the active ingredient in Actifed® Cold & Allergy Tablets
24 TABLETS
* This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Actifed® Cold & Allergy Tablets.
50844 REV1010C52508
Distributed by:
Great Lakes Wholesale & Marketing L.L.C.
3729 Patterson Ave., S.E.
Grand Rapids, MI 49512
www.glwholesale.com
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Allerfed Cold and AllergyChlorpheniramine maleate and Phenylephrine HCl TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||