Allegra-D 24 Hour Allergy and Congestion
Allegra-D 24 Hour Allergy and Congestion
FULL PRESCRIBING INFORMATION
Drug Facts
( in each tablet)
Fexofenadine HCI 180 mg
Antihistamine
( in each tablet)
Pseudoephedrine HCI 240 mg
Nasal decongestant
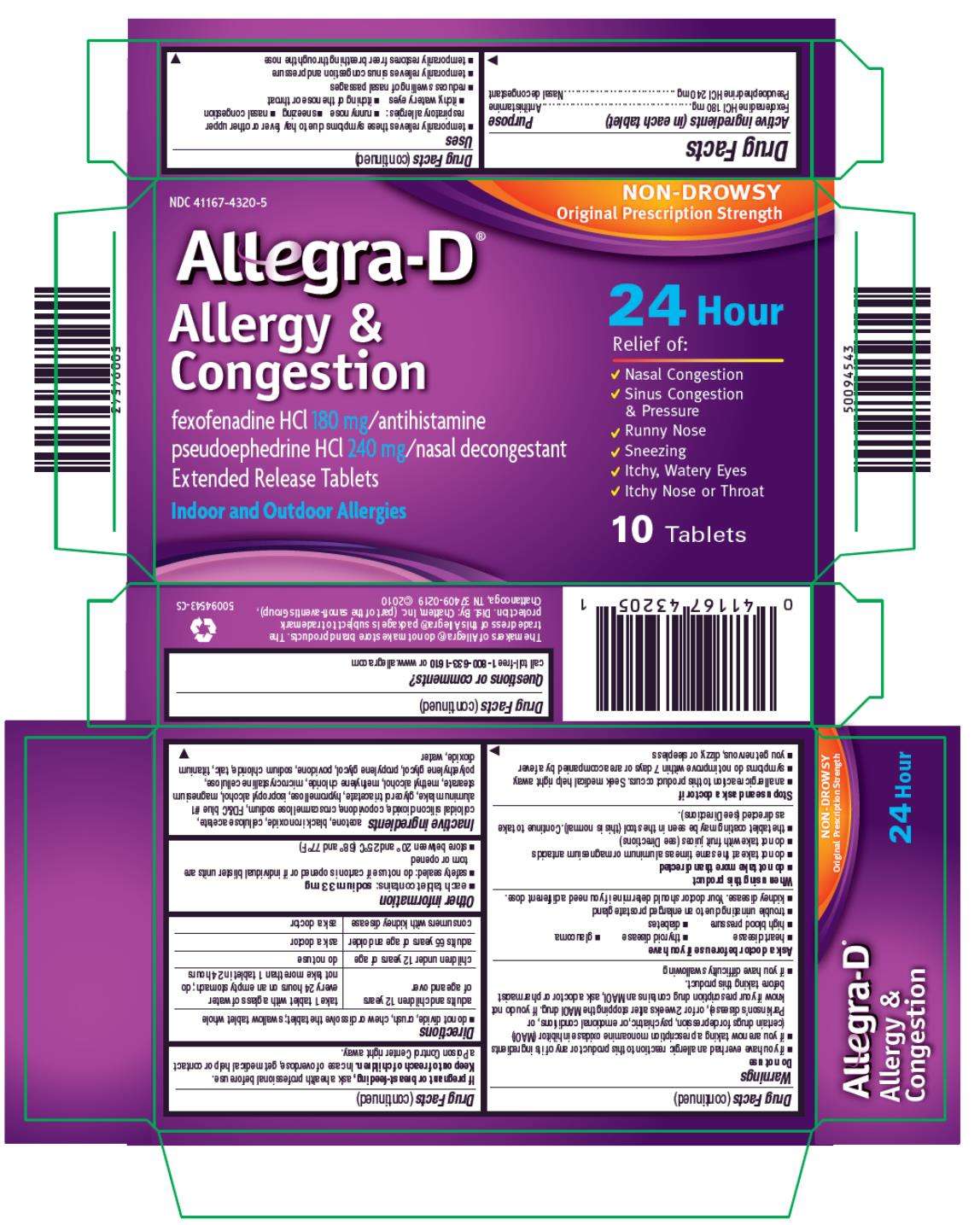
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
▪ runny nose
▪ sneezing
▪ nasal congestion
▪ itchy, watery eyes
▪ itching of the nose or throat
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI)
(certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- If you have difficulty swallowing
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
-
do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- the tablet coating may be seen in the stool (this is normal). Continue to take as directed (see Directions).
- an allergic reaction to this product occurs. Seek medical help right away.
- symptoms do not improve within 7 days or are accompanied by a fever
- you get nervous, dizzy, or sleepless
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
- do not divide, crush, chew or dissolve the tablet; swallow tablet whole
| adults and children 12 years of age and over |
take 1 tablet with a glass of water every 24 hours on an empty stomach; do not take more than 1 tablet in 24 hours |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
- each tablet contains: sodium 33 mg
- safety sealed: do not use if carton is opened or if individual blister units are torn or opened
- store between 20º and 25ºC (68º and 77ºF)
acetone, black iron oxide, cellulose acetate, colloidal silicon dioxide, copovidone, croscarmellose sodium, FD&C blue #1 aluminum lake, glycerol triacetate, hypromellose, isopropyl alcohol, magnesium stearate, methyl alcohol, methylene chloride, microcrystalline cellulose, polyethylene glycol, propylene glycol, povidone, sodium chloride, talc, titanium dioxide, water
call toll-free 1-800-633-1610 or www.allegra.com
NDC 41167-4320-3
NON-DROWSY
ORIGINAL PRESCRIPTION STRENGTH
Allegra-D®
Allergy & Congestion
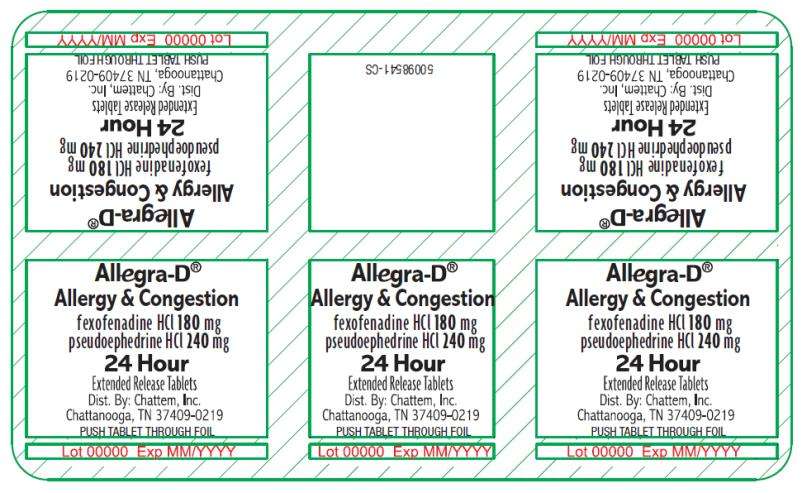
fexofenadine HCI 180 mg/antihistamine
pseudoephedrine HCI 240 mg/nasal decongestant
Extended Release Tablets
5 Tablets

NDC 41167-4320-5
NON-DROWSY
ORIGINAL PRESCRIPTION STRENGTH
Allegra-D®
Allergy & Congestion
fexofenadine HCI 180 mg/antihistamine
pseudoephedrine HCI 240 mg/nasal decongestant
Extended Release Tablets
10 Tablets
NDC 41167-4320-7
NON-DROWSY
ORIGINAL PRESCRIPTION STRENGTH
Allegra-D®
Allergy & Congestion
fexofenadine HCI 180 mg/antihistamine
pseudoephedrine HCI 240 mg/nasal decongestant
Extended Release Tablets
15 Tablets

Allegra-D®
Allergy & Congestion
fexofenadine HCI 180 mg/antihistamine
pseudoephedrine HCI 240 mg/nasal decongestant
24 Hour
Extended Release Tablets
5ct Blister Pack
Allegra-D 24 Hour Allergy and Congestionfexofenadine hydrochloride and pseudoephedrine hydrochloride TABLET, FILM COATED, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||