CBI Laboratories, Inc
Moisture Defense SPF 15
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
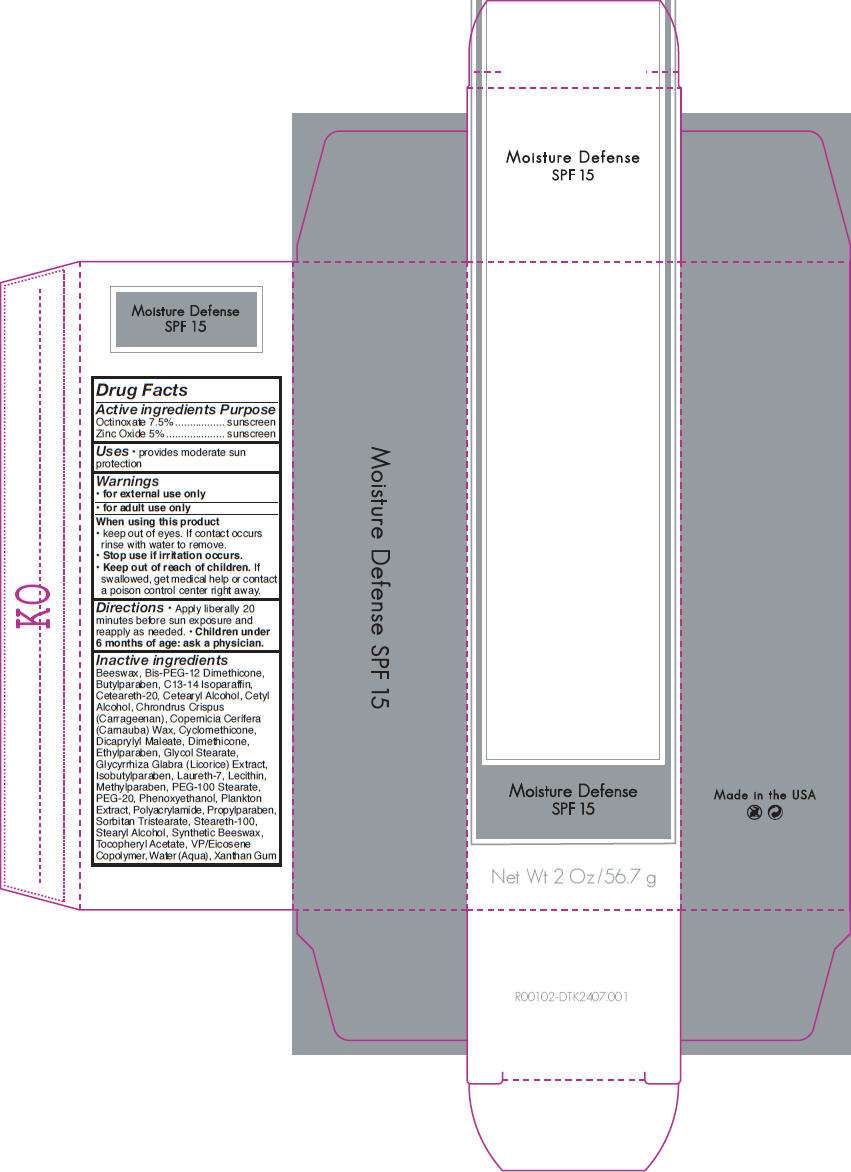
Drug Facts
Active ingredient
Purpose
|
Active ingredients
|
Purpose
|
| Octinoxate 7.5% |
sunscreen |
| Zinc Oxide 5% |
sunscreen |
Advanced Protection Uses
- provides moderate sun protection
Warnings
-
for external use only
-
for adult use only
When using this product
- keep out of eyes. If contact occurs rinse with water to remove.
-
Stop use if irritation occurs.
-
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Directions
- Apply liberally 20 minutes before sun exposure and reapply as needed.
-
Children under 6 months of age: ask a physician.
Inactive ingredients
Beeswax, Bis-PEG-12 Dimethicone, Butylparaben, C13-14 Isoparaffin, Ceteareth-20, Cetearyl Alcohol, Cetyl Alcohol, Chrondrus Crispus (Carrageenan), Copernicia Cerifera (Carnauba) Wax, Cyclomethicone, Dicaprylyl Maleate, Dimethicone, Ethylparaben, Glycol Stearate, Glycyrrhiza Glabra (Licorice) Extract, Isobutylparaben, Laureth-7, Lecithin, Methylparaben, PEG-100 Stearate, PEG-20, Phenoxyethanol, Plankton Extract, Polyacrylamide, Propylparaben, Sorbitan Tristearate, Steareth-100, Stearyl Alcohol, Synthetic Beeswax, Tocopheryl Acetate, VP/Eicosene Copolymer, Water (Aqua), Xanthan Gum
PRINCIPAL DISPLAY PANEL - 56.7 g Carton
Moisture Defense
SPF 15
Net Wt 2 Oz/56.7 g
Advanced Protection
OCTINOXATE and Zinc Oxide CREAM
Product Information
|
|
Product Type
|
Human otc drug label |
Item Code (Source)
|
NDC:24623-001 |
|
Route of Administration
|
TOPICAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
OCTINOXATE OCTINOXATE |
|
0.075 g
|
|
Zinc Oxide Zinc oxide |
|
0.05 g
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:24623-001-20 |
56.7 in 1 TUBE |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
part |
part352 |
2001-08-25 |
|
|
