Advanced Lightning
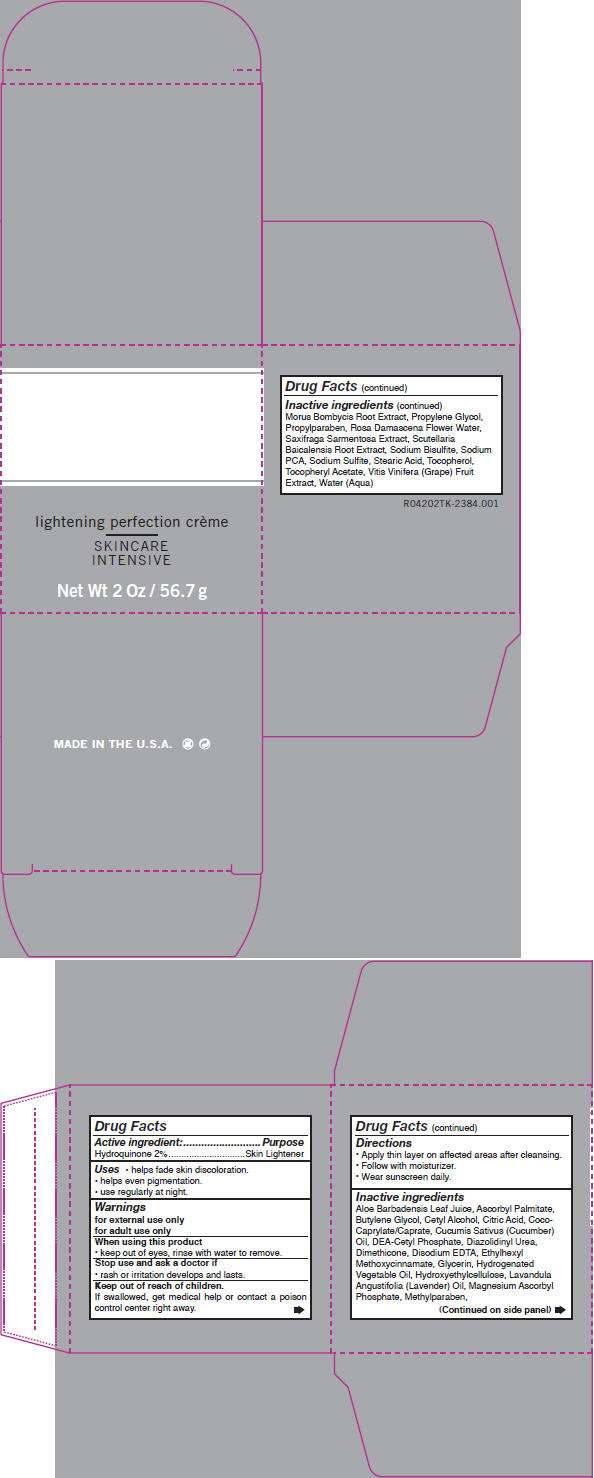
lightening perfection crème SKINCARE INTENSIVE
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Advanced Lightning Uses
- Warnings
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL - 56.7 g Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Hydroquinone 2%
Purpose
Skin Lightener
Advanced Lightning Uses
- helps fade skin discoloration.
- helps even pigmentation.
- use regularly at night.
Warnings
for external use only
for adult use only
When using this product
- keep out of eyes, rinse with water to remove.
Stop use and ask a doctor if
- rash or irritation develops and lasts.
Keep out of reach of children.
If swallowed, get medical help or contact a poison control center right away.
Directions
- Apply thin layer on affected areas after cleansing.
- Follow with moisturizer.
- Wear sunscreen daily.
Inactive ingredients
Aloe Barbadensis Leaf Juice, Ascorbyl Palmitate, Butylene Glycol, Cetyl Alcohol, Citric Acid, Coco-Caprylate/Caprate, Cucumis Sativus (Cucumber) Oil, DEA-Cetyl Phosphate, Diazolidinyl Urea, Dimethicone, Disodium EDTA, Ethylhexyl Methoxycinnamate, Glycerin, Hydrogenated Vegetable Oil, Hydroxyethylcellulose, Lavandula Angustifolia (Lavender) Oil, Magnesium Ascorbyl Phosphate, Methylparaben, Morus Bombycis Root Extract, Propylene Glycol, Propylparaben, Rosa Damascena Flower Water, Saxifraga Sarmentosa Extract, Scutellaria Baicalensis Root Extract, Sodium Bisulfite, Sodium PCA, Sodium Sulfite, Stearic Acid, Tocopherol, Tocopheryl Acetate, Vitis Vinifera (Grape) Fruit Extract, Water (Aqua)
PRINCIPAL DISPLAY PANEL - 56.7 g Carton
lightening perfection crème
SKINCARE
INTENSIVE
Net Wt 2 Oz / 56.7 g
Advanced LightningOCTINOXATE and HYDROQUINONE CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||