Acyclovir
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACYCLOVIR DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ACYCLOVIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ACYCLOVIR ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ACYCLOVIR DESCRIPTION
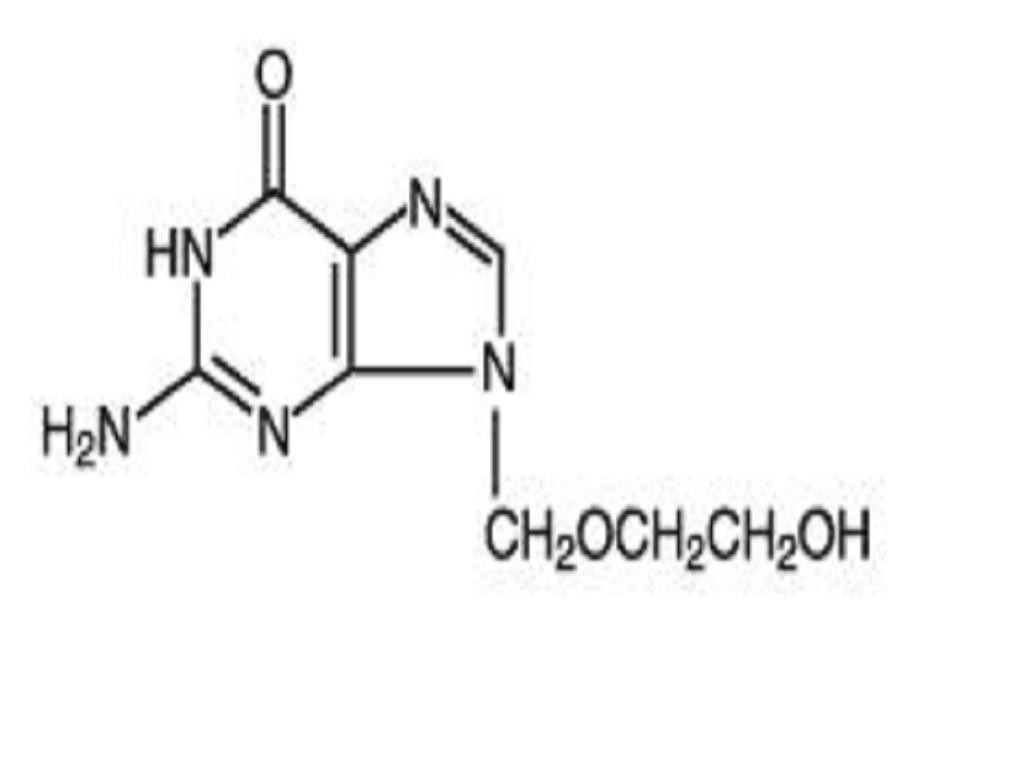
VIROLOGY
Mechanism of Antiviral Action
Antiviral Activities
Drug Resistance
CLINICAL PHARMACOLOGY
Pharmacokinetics**
Special Populations
Adults With Impaired Renal Function
Geriatrics
Pediatrics
Drug Interactions
Clinical Trials
Initial Genital Herpes
Recurrent Genital Herpes
Herpes Zoster Infections
Chickenpox
INDICATIONS & USAGE
INDICATIONS AND USAGEHerpes Zoster Infections
Genital Herpes
Chickenpox
ACYCLOVIR CONTRAINDICATIONS
CONTRAINDICATIONSWARNINGS
WARNINGSPRECAUTIONS
INFORMATION FOR PATIENTS
Information for PatientsHerpes Zoster
Genital Herpes Infections
Chickenpox
DRUG INTERACTIONS
Drug InteractionsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis, Impairment of FertilityPREGNANCY
Teratogenic EffectsPregnancy category B
NURSING MOTHERS
Nursing MothersPEDIATRIC USE
Pediatric UseGERIATRIC USE
Geriatric UseACYCLOVIR ADVERSE REACTIONS
ADVERSE REACTIONSHerpes Simplex
Short-Term Administration
Long-Term Administration
Herpes Zoster
Chickenpox
Observed During Clinical Practice
General
Nervous
Digestive
Hematologic and Lymphatic
Hepatobiliary Tract and Pancreas
Musculoskeletal
Skin
Special Senses
Urogenital
OVERDOSAGE
OVERDOSAGEDOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATIONAcute Treatment of Herpes Zoster
Genital Herpes
Treatment of Initial Genital Herpes
Chronic Suppressive Therapy for Recurrent Disease
Intermittent Therapy
Treatment of Chickenpox
Children (2 Years of age and Older)
Adults and Children Over 40 kg
Patients With Acute or Chronic Renal Impairment
Table 3: Dosage Modification for Renal Impairment
Hemodialysis
Peritoneal Dialysis
Bioequivalence of Dosage Forms
STORAGE AND HANDLING
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:STARCH, CORN
LACTOSE MONOHYDRATE
MAGNESIUM STEARATE
SODIUM LAURYL SULFATE
GELATIN
FD&C BLUE NO. 1
D&C RED NO. 28
D&C RED NO. 33
TITANIUM DIOXIDE
FD&C BLUE NO. 2
FD&C RED NO. 40
D&C YELLOW NO. 10
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

AcyclovirAcyclovir CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!