Acyclovir
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACYCLOVIR DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ACYCLOVIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ACYCLOVIR ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ACYCLOVIR DESCRIPTION
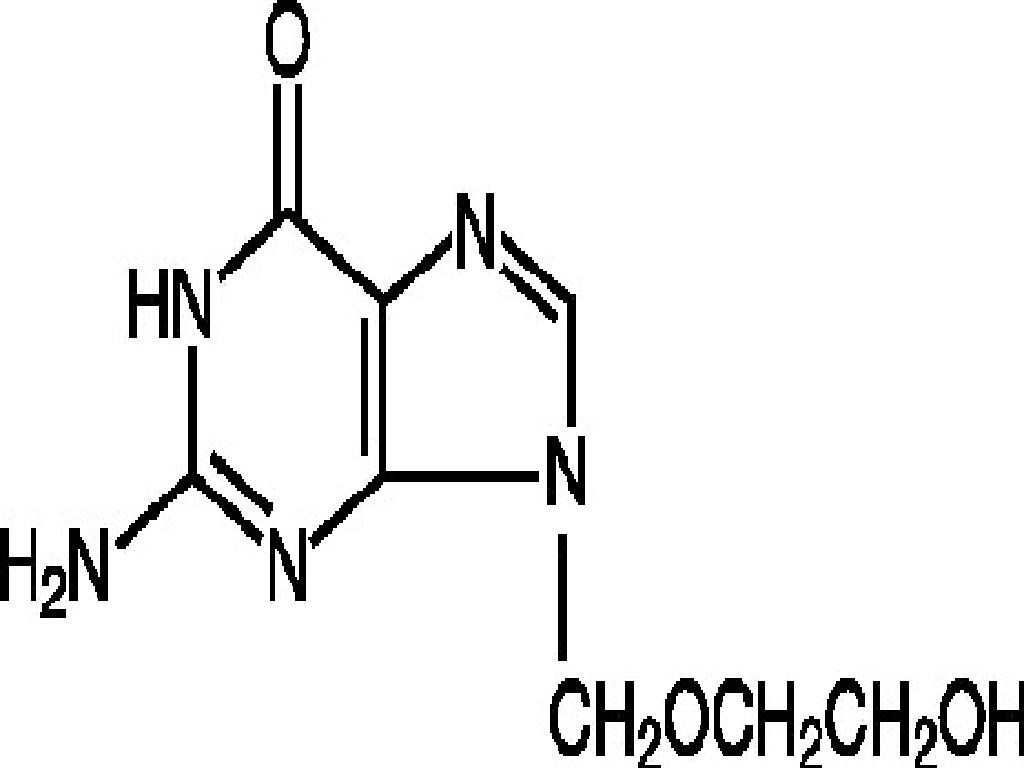
VIROLOGY
Mechanism of Antiviral Action
Antiviral Activities
Drug Resistance
CLINICAL PHARMACOLOGY
Pharmacokinetics**
Special Populations
Adults With Impaired Renal Function
Geriatrics
Pediatrics
Drug Interactions
Clinical Trials
Initial Genital Herpes
Recurrent Genital Herpes
Herpes Zoster Infections
Chickenpox
INDICATIONS & USAGE
Herpes Zoster InfectionsGenital Herpes
Chickenpox
ACYCLOVIR CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
INFORMATION FOR PATIENTS
Herpes Zoster
Genital Herpes Infections
Chickenpox
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy category B
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
ACYCLOVIR ADVERSE REACTIONS
Herpes SimplexShort-Term Administration
Long-Term Administration
Herpes Zoster
Chickenpox
Observed During Clinical Practice
General
Nervous
Digestive
Hematologic and Lymphatic
Hepatobiliary Tract and Pancreas
Musculoskeletal
Skin
Special Senses
Urogenital
OVERDOSAGE
DOSAGE & ADMINISTRATION
Acute Treatment of Herpes ZosterGenital Herpes
Treatment of Initial Genital Herpes
Chronic Suppressive Therapy for Recurrent Disease
Intermittent Therapy
Treatment of Chickenpox
Children (2 Years of age and Older)
Adults and Children Over 40 kg
Patients With Acute or Chronic Renal Impairment
Hemodialysis
Peritoneal Dialysis
Bioequivalence of Dosage Forms
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

AcyclovirAcyclovir CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!