Acyclovir
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACYCLOVIR DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ACYCLOVIR CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ACYCLOVIR ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
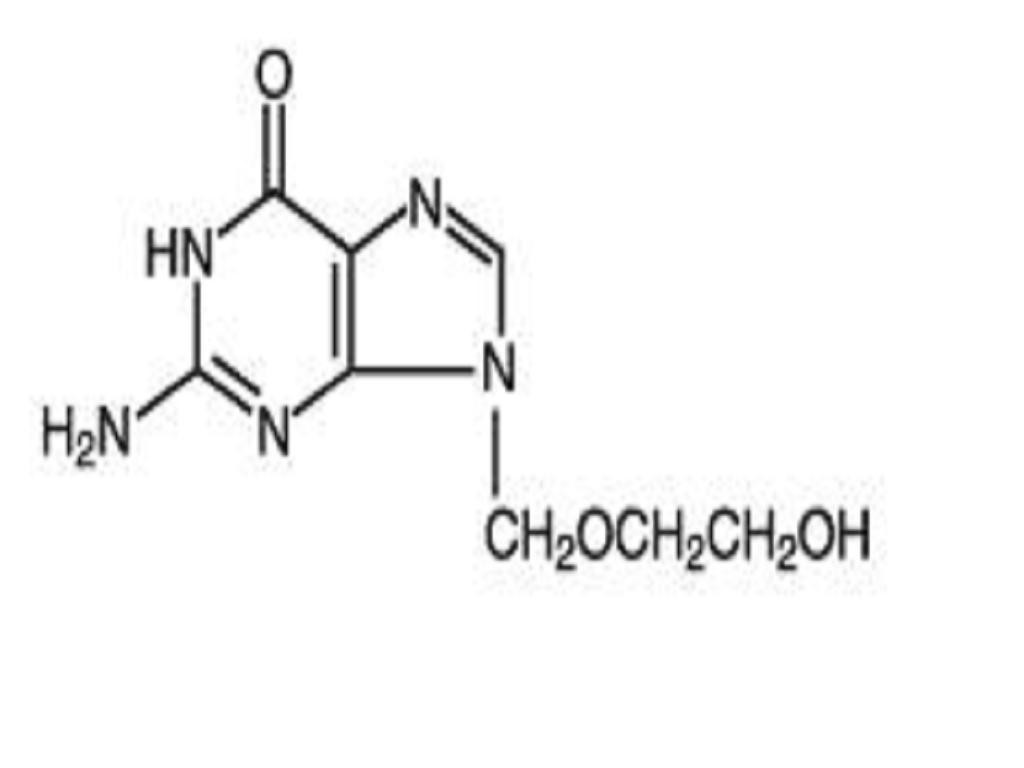
ACYCLOVIR DESCRIPTION
VIROLOGY
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
Initial Genital Herpes:Recurrent Genital Herpes:
Herpes Zoster Infections:
Chickenpox:
INDICATIONS & USAGE
Herpes Zoster Infections:Genital Herpes:
Chickenpox:
ACYCLOVIR CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
Information for Patients:
Herpes Zoster:
Genital Herpes Infections:
Chickenpox:
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
ACYCLOVIR ADVERSE REACTIONS
Herpes Simplex:Long-Term Administration:
Herpes Zoster:
Chickenpox:Observed During Clinical Practice:
General:
Nervous:
Digestive:
Hematologic and Lymphatic:
Hepatobiliary Tract and Pancreas:
Skin:
Special Senses:
Urogenital:
OVERDOSAGE
DOSAGE & ADMINISTRATION
Acute Treatment of Herpes Zoster:Genital Herpes:
Chronic Suppressive Therapy for Recurrent Disease:
Intermittent Therapy:
Treatment of Chickenpox:
Adults and Children over 40 kg:
Hemodialysis:
Peritoneal Dialysis:
Bioequivalence of Dosage Forms:
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
AcyclovirAcyclovir CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!