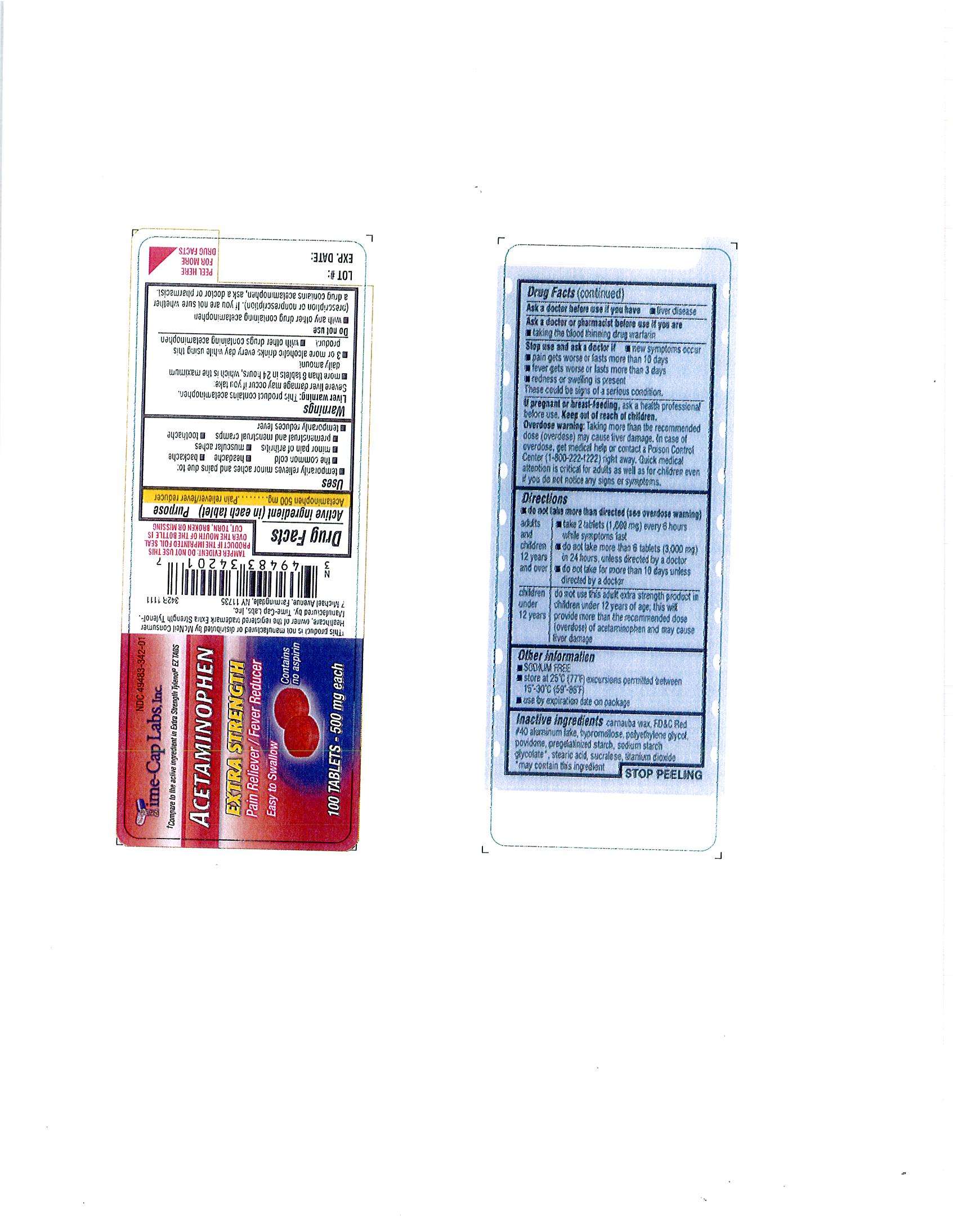
ACETAMINOPHEN
FULL PRESCRIBING INFORMATION
Active ingredient
Acetaminophen 500 mg
Purpose
PURPOSE: Pain Reliever - fever reducer
Keep Out of the Reach of Children: In case of overdose, get medical help or contact a Poison Control Center right away
Uses
INDICATIONS AND USAGE:
Pain Reliever – temporarily relieves minor aches and pains due to: the common cold, headache, backache, muscular aches, toothache, premenstrual and menstrual cramps, minor pain of arthritis. Temporarily reduces fever.
Warnings;
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:more than 8 tablets in 24 hours, which is the maximum daily amount; 3 or more alcoholic drinks every day while using this product; with other drugs containing acetaminophen.
Do not take more than directed (see overdosage warning)
Adults and children 12 years and over: take 2 tablets (1,000 mg) every 6 hours while symptoms last; do not take more than 6 tablets (3,000 mg) in 24 hours, unless directed by a doctor; do not take for more than 10 days unless directed by a doctor
CARNAUBA WAX, FD-C RED NO. 40 ALUMINUM LAKE, HYPROMELLOSE, POLYETHYLENE GLYCOL(PEG) 400, POLYETHYLENE GLYCOL (peg) 8000, POVIDONE, PREGELATINIZED STARCH, SODIUM STARCH GLYCOLATE**, STEARIC ACID, SUCRALOSE, TITANIUM DIOXIDE
** MAY CONTAIN THIS INGREDIENT
ACETAMINOPHENACETAMINOPHEN TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||