Ribasphere
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- RIBASPHERE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- RIBASPHERE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- RIBASPHERE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- INFORMATION FOR PATIENTS
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
RIBASPHERE (ribavirin, USP) monotherapy is not effective for the treatment of chronic hepatitis C virus infection and should not be used alone for this indication (seeWARNINGS).The primary clinical toxicity of ribavirin is hemolytic anemia. The anemia associated with ribavirin therapy may result in worsening of cardiac disease that has led to fatal and nonfatal myocardial infarctions. Patients with a history of significant or unstable cardiac disease should not be treated with ribavirin (seeWARNINGS,ADVERSE REACTIONS, andDOSAGE AND ADMINISTRATION).
Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin. In addition, ribavirin has a multiple dose half-life of 12 days, and it may persist in non-plasma compartments for as long as 6 months. Ribavirin therapy is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Extreme care must be taken to avoid pregnancy during therapy and for 6 months after completion of therapy in both female patients and in female partners of male patients who are taking ribavirin therapy. At least two reliable forms of effective contraception must be utilized during treatment and during the 6-month posttreatment follow-up period (seeCONTRAINDICATIONS,WARNINGS, andPRECAUTIONS: Information for Patients, and Pregnancy: Category X).
RIBASPHERE DESCRIPTION
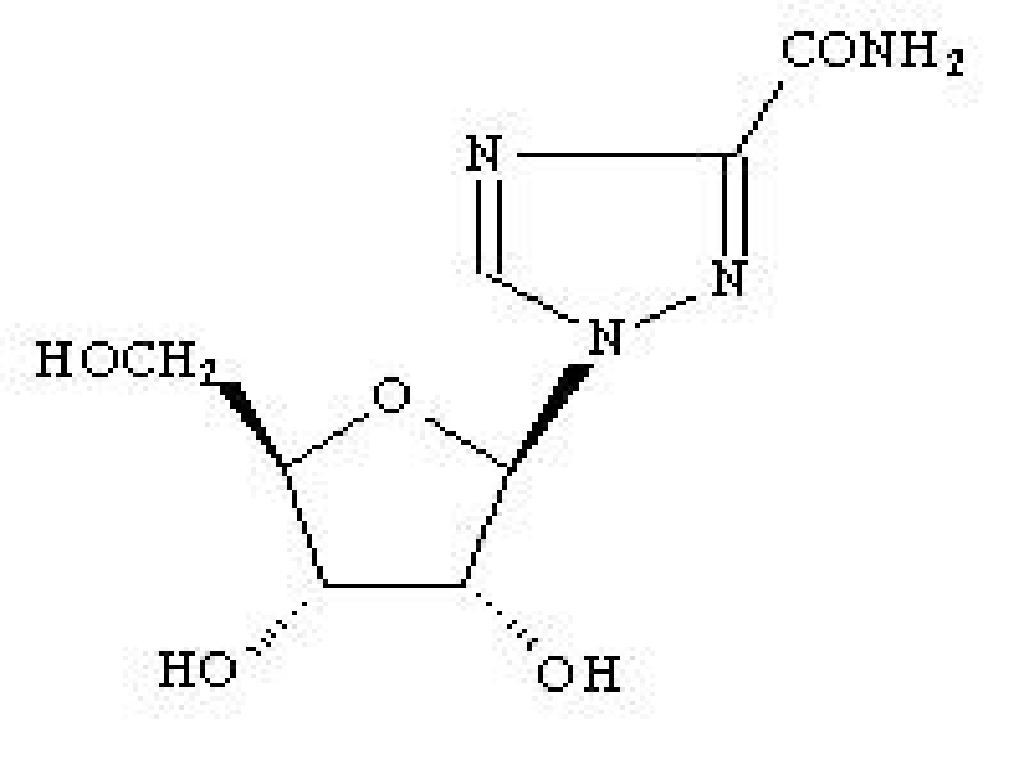
Mechanism of Action
CLINICAL PHARMACOLOGY
PharmacokineticsPRECAUTIONSDOSAGE AND ADMINISTRATION
Elimination and Metabolism
Special Populations
Race
Renal Dysfunction
WARNINGSDOSAGE AND ADMINISTRATION
Hepatic Impairment
Pediatric Patients
Elderly Patients
Gender
Drug Interactions
Nucleoside Analogues
PRECAUTIONS: Drug Interactions
Drugs Metabolized by Cytochrome P450
PRECAUTIONS: Drug Interactions
CLINICAL STUDIES
HCV PatientsTable 1
HCV Genotypes
Table 2
Other Treatment Response Predictors
INDICATIONS & USAGE
RIBASPHERE CONTRAINDICATIONS
-
● Patients with known hypersensitivity to RIBASPHERE (ribavirin, USP) or to any component of the tablet.
-
● Women who are pregnant.
-
● Men whose female partners are pregnant.
-
● Patients with hemoglobinopathies (e.g., thalassemia major or sickle-cell anemia).
-
● Autoimmune hepatitis.
-
● Hepatic decompensation (Child-Pugh score greater than 6; class B and C) in cirrhotic CHC monoinfected patients before or during treatment.
WARNINGS
RIBASPHERE(ribavirin, USP) must not be used alone because ribavirin monotherapy is not effective for the treatment of chronic hepatitis C virus infection. The safety and efficacy of ribavirin have only been established when used together with peginterferon alfa-2a, recombinant.There are significant adverse events caused by ribavirin/peginterferon alfa-2a therapy, including severe depression and suicidal ideation, hemolytic anemia, suppression of bone marrow function, autoimmune and infectious disorders, pulmonary dysfunction, pancreatitis, and diabetes. The PEGASYS package insert and MEDICATION GUIDE should be reviewed in their entirety prior to initiation of combination treatment for additional safety information.
General
Pregnancy
Ribavirin may cause birth defects and/or death of the exposed fetus. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. Ribavirin has demonstrated significant teratogenic and/or embryocidal effects in all animal species in which adequate studies have been conducted. These effects occurred at doses as low as one twentieth of the recommended human dose of ribavirin. RIBASPHERE (ribavirin, USP) THERAPY SHOULD NOT BE STARTED UNLESS A REPORT OF A NEGATIVE PREGNANCY TEST HAS BEEN OBTAINED IMMEDIATELY PRIOR TO PLANNED INITIATION OF THERAPY. Patients should be instructed to use at least two forms of effective contraception during treatment and for 6 months after treatment has been stopped. Pregnancy testing should occur monthly during RIBASPHERE (ribavirin, USP) therapy and for 6 months after therapy has stopped (seeCONTRAINDICATIONSandPRECAUTIONS: Information for Patientsand Pregnancy: Category X).
Anemia
The primary toxicity of ribavirin is hemolytic anemia (hemoglobin <10 g/dL), which was observed in approximately 13% of all ribavirin and peginterferon alfa-2a treated patients in clinical trials (seePRECAUTIONS: Laboratory Tests). The anemia associated with ribavirin occurs within 1 to 2 weeks of initiation of therapy. BECAUSE THE INITIAL DROP IN HEMOGLOBIN MAY BE SIGNIFICANT, IT IS ADVISED THAT HEMOGLOBIN OR HEMATOCRIT BE OBTAINED PRETREATMENT AND AT WEEK 2 AND WEEK 4 OF THERAPY OR MORE FREQUENTLY IF CLINICALLY INDICATED. Patients should then be followed as clinically appropriate.
Fatal and nonfatal myocardial infarctions have been reported in patients with anemia caused by ribavirin. Patients should be assessed for underlying cardiac disease before initiation of ribavirin therapy. Patients with pre-existing cardiac disease should have electrocardiograms administered before treatment, and should be appropriately monitored during therapy. If there is any deterioration of cardiovascular status, therapy should be suspended or discontinued (seeDOSAGE AND ADMINISTRATION: RIBASPHERE (ribavirin, USP) Dosage Modification Guidelines). Because cardiac disease may be worsened by drug induced anemia, patients with a history of significant or unstable cardiac disease should not use RIBASPHERE (ribavirin, USP) (seeADVERSE REACTIONS).
Hepatic Failure
CONTRAINDICATIONS
Hypersensitivity
ADVERSE REACTIONS: Postmarketing Experience
Pulmonary
Other
CLINICAL PHARMACOLOGY: Special Populations
PRECAUTIONS
INFORMATION FOR PATIENTS
CONTRAINDICATIONSWARNINGS
ADVERSE REACTIONSLaboratory Tests
LABORATORY TESTS
-
● Platelet countcells/mm3 (as low as 75,000 cells/mm3 in patients with cirrhosis)
-
● Absolute neutrophil count (ANC)cells/mm3
-
● TSH and T4 within normal limits or adequately controlled thyroid function
-
● ECG (seeWARNINGS)
-
● Hemoglobing/dL for women andg/dL for men in CHC monoinfected patients
DRUG INTERACTIONS
Nucleoside Analogues
WARNINGSPRECAUTIONSDOSAGE AND ADMINISTRATION: Dose Modifications
CLINICAL PHARMACOLOGY: Drug Interactions
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
CONTRAINDICATIONSNURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
CLINICAL PHARMACOLOGY: Special PopulationsEffect of Gender
RIBASPHERE ADVERSE REACTIONS
WARNINGSBecause clinical trials are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug. Also, the adverse event rates listed here may not predict the rates observed in a broader patient population in clinical practice.
Laboratory Test Values
DOSAGE AND ADMINISTRATION: Dose Modifications
Postmarketing Experience
WARNINGS: Hypersensitivity
OVERDOSAGE
DOSAGE & ADMINISTRATION
CHC Monoinfection
Table 4
Table 4
Table 2
Dose Modifications
If severe adverse reactions or laboratory abnormalities develop during combination RIBASPHERE (ribavirin, USP)/peginterferon alfa-2a therapy, the dose should be modified or discontinued, if appropriate, until the adverse reactions abate. If intolerance persists after dose adjustment, RIBASPHERE (ribavirin, USP)/peginterferon alfa-2a therapy should be discontinued.
Table 5WARNINGS
Renal Impairment
WARNINGSCLINICAL PHARMACOLOGY: Special Populations
HOW SUPPLIED
STORAGE AND HANDLING
INFORMATION FOR PATIENTS
Pregnancy
Anemia
Boxed Warning
SPL MEDGUIDE
FDA-approved Medication GuideRIBASPHERE
(ribavirin, USP)
Tablets
What is the most important information I should know about Ribasphere (ribavirin, USP)?
1. You should not take Ribasphere (ribavirin, USP) alone to treat chronic hepatitis C infection.
2. Ribasphere (ribavirin, USP) may cause you to have a blood problem (hemolytic anemia) that can worsen any heart problems you have, and cause you to have a heart attack or die.
3. Ribasphere (ribavirin, USP) may cause birth defects or death of your unborn baby.
-
● Females must have a pregnancy test before starting Ribasphere (ribavirin, USP), every month while treated with Ribasphere (ribavirin, USP), and every month for the 6 months after treatment with Ribasphere (ribavirin, USP).
-
● If you or your female sexual partner becomes pregnantwhile taking Ribasphere (ribavirin, USP) or within 6 months after you stop taking Ribasphere (ribavirin, USP), tell your healthcare provider right away. You or your healthcare provider should contact theRibavirin Pregnancy Registry by calling 1-800-593-2214.The Ribavirin Pregnancy Registry collects information about what happens to mothers and their babies if the mother takes Ribasphere (ribavirin, USP) while she is pregnant.
Who should not take Ribasphere (ribavirin, USP)?
SeeWhat is the most important information I should know about Ribasphere (ribavirin, USP)?
Do not take Ribasphere (ribavirin, USP) if you:
-
● have certain types of hepatitiscaused by your immune system attacking your liver (autoimmune hepatitis)
-
● have certain blood disorders, such as thalassemia major or sickle-cell anemia (hemoglobinopathies)
-
● have severe kidney disease
-
● take didanosine(Videx or Videx EC)
What should I tell my healthcare provider before taking Ribasphere (ribavirin, USP)?
Before you take Ribasphere (ribavirin, USP), tell your healthcare provider if you have or have had:
-
● treatment for hepatitis C that did not work for you
-
● serious allergic reactions to Ribasphere (ribavirin, USP) or to any of the ingredients in Ribasphere (ribavirin, USP).See the end of this Medication Guide for a list of ingredients.
-
● breathing problems.Ribasphere (ribavirin, USP) may cause or worsen your breathing problems you already have.
-
● vision problems.Ribasphere (ribavirin, USP) may cause eye problems or worsen eye problems you already have. You should have an eye exam before you start treatment with Ribasphere (ribavirin, USP).
-
● certain blood disorders such as anemia
-
● high blood pressure, heart problems or have had a heart attack.Your healthcare provider should test your blood and heart before you start treatment with Ribasphere (ribavirin, USP).
-
● thyroid problems
-
● diabetes.Ribasphere (ribavirin, USP) and peginterferon alfa-2a combination therapy may make your diabetes worse or harder to treat.
-
● liver problemsother than hepatitis C virus infection
-
● human immunodeficiency virus (HIV) or other immunity problems
-
● mental health problems,including depression or thoughts of suicide
-
● kidney problems
-
● an organ transplant
-
● drug addiction or abuse
-
● infection with hepatitis B virus
-
● any other medical condition
-
● are breast-feeding.It is not known if Ribasphere (ribavirin, USP) passes into your breast milk. You and your healthcare provider should decide if you will take Ribasphere (ribavirin, USP) or breast-feed.
How should I take Ribasphere (ribavirin, USP)?
-
● Take Ribasphere (ribavirin, USP) exactly as your healthcare provider tells you. Your healthcare provider will tell you how much Ribasphere (ribavirin, USP) to take and when to take it.
-
● Take Ribasphere (ribavirin, USP) with food.
-
● If you miss a dose of Ribasphere (ribavirin, USP), take the missed dose as soon as possible during the same day. Do not double the next dose. If you have questions about what to do, call your healthcare provider.
-
● If you take too much Ribasphere (ribavirin, USP), call your healthcare provider or local Poison Control Center right away, or go to the nearest hospital emergency room right away.
-
● Your healthcare provider should do blood tests before you start treatment with Ribasphere (ribavirin, USP), at weeks 2 and 4 of treatment, and then as needed to see how well you are tolerating treatment and to check for side effects. Your healthcare provider may change your dose of Ribasphere (ribavirin, USP) based on blood test results or side effects you may have.
-
● If you have heart problems, your healthcare provider should check your heart by doing an electrocardiogram before you start treatment with Ribasphere (ribavirin, USP), and if needed during treatment.
-
● Ribasphere (ribavirin, USP) can make you feel tired, dizzy, or confused. You should not drive or operate machinery if you have any of these symptoms.
-
● Do not drink alcohol, including beer, wine, and liquor. This may make your liver disease worse.
Ribasphere (ribavirin, USP) may cause serious side effects including:
SeeWhat is the most important information I should know about Ribasphere (ribavirin, USP)?
-
● Swelling and irritation of your pancreas (pancreatitis).You may have stomach pain, nausea, vomiting or diarrhea.
-
● Severe allergic reactions. Symptoms may include hives, wheezing, trouble breathing, chest pain, swelling of your mouth, tongue, or lips, or severe rash.
-
● Serious breathing problems.Difficulty breathing may be a sign of a serious lung infection (pneumonia) that can lead to death.
-
● Serious eye problemsthat may lead to vision loss or blindness.
-
● Liver problems.Some people may get worsening of liver function. Tell your healthcare provider right away if you have any of these symptoms: stomach bloating, confusion, brown urine, and yellow eyes.
-
● Severe depression
-
● Suicidal thoughts and attempts
Common side effects of Ribasphere (ribavirin, USP) taken with peginterferon alfa-2a include:
-
● flu-like symptoms-feeling tired, headache, shaking along with high temperature (fever), and muscle or joint aches
-
● mood changes, feeling irritable, anxiety, and difficulty sleeping
-
● loss of appetite, nausea, vomiting, and diarrhea
-
● hair loss
-
● itching
How should I store Ribasphere (ribavirin, USP)?
-
● Store Ribasphere (ribavirin, USP) tablets between 59and 86(15and 30
-
● Keep the bottle tightly closed.
General information about the safe and effective use of Ribasphere (ribavirin, USP)
What are the ingredients in Ribasphere (ribavirin, USP) ?
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

RibasphereRibavirin TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!