Lisinopril and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
USE IN PREGNANCYWhen used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. When pregnancy is detected, lisinopril and hydrochlorothiazide should be discontinued as soon as possible. SeeWARNINGS,Pregnancy,Lisinopril, Fetal/Neonatal Morbidity and Mortality.
LISINOPRIL AND HYDROCHLOROTHIAZIDE DESCRIPTION
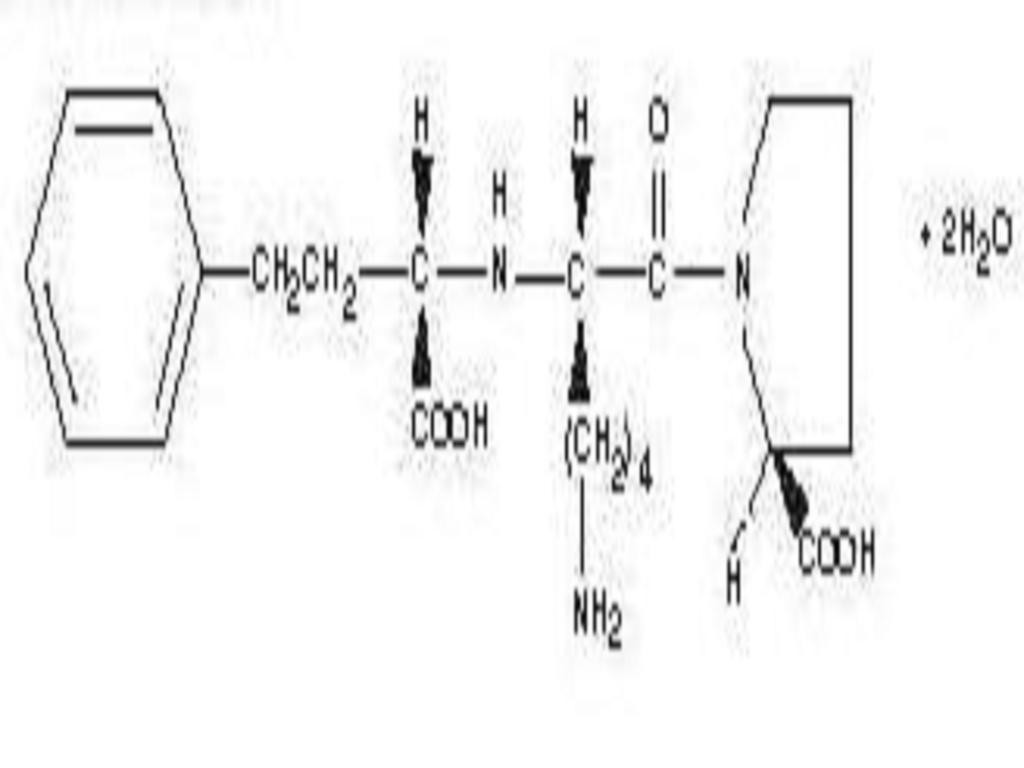

CLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
PRECAUTIONS
DOSAGE AND ADMINISTRATION
WARNINGS
PRECAUTIONS
INDICATIONS & USAGE
DOSAGE AND ADMINISTRATION
WARNINGS
WARNINGSLisinopril
LISINOPRIL AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
WARNINGS
LisinoprilADVERSE REACTIONS
INDICATIONS AND USAGECONTRAINDICATIONS
Anaphylactoid Reactions During Desensitization:
Anaphylactoid Reactions During Membrane Exposure:
Hypotension and Related Effects
PRECAUTIONSDrug InteractionsADVERSE REACTIONS
PRECAUTIONSDrug InteractionsADVERSE REACTIONSDOSAGE AND ADMINISTRATION
Leukopenia/Neutropenia/Agranulocytosis:
Hepatic Failure:
Pregnancy
Lisinopril, Fetal/Neonatal Morbidity and Mortality
Lisinopril
Fetal/Neonatal Morbidity and Mortality:
Hydrochlorothiazide
Teratogenic Effects:
Nonteratogenic Effects:
Hydrochlorothiazide
PRECAUTIONSDrug Interactions, LisinoprilHydrochlorothiazide
PRECAUTIONS
GeneralAortic Stenosis/Hypertrophic Cardiomyopathy:
Impaired Renal Function:
DOSAGE AND ADMINISTRATION
Hyperkalemia:
Drug Interactions
Cough:
Surgery/Anesthesia:
Drug Interactions, Agents Increasing Serum Potassium
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
LisinoprilWARNINGSDOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATION
Agents Increasing Serum Potassium:
Lithium:
Hydrochlorothiazide
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
WARNINGSPregnancyLisinopril, Fetal/Neonatal Morbidity and Mortality
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
LISINOPRIL AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
WARNINGS
WARNINGS
WARNINGS
PRECAUTIONSCough
PRECAUTIONS
PRECAUTIONS
PRECAUTIONS
WARNINGSHepatic Failure
WARNINGS Anaphylactoid Reactions During Membrane ExposureWARNINGS, HypotensionWARNINGS, Hepatic FailurePRECAUTIONSandDOSAGE AND ADMINISTRATION), pyelonephritis, dysuria, breast pain.
Miscellaneous: A symptom complex has been reported which may include a positive ANA, an elevated erythrocyte sedimentation rate, arthralgia/arthritis, myalgia, fever, vasculitis, eosinophilia and leukocytosis. Rash, photosensitivity or other dermatological manifestations may occur alone or in combination with these symptoms.
Fetal/Neonatal Morbidity and Mortality: SeeWARNINGS,Pregnancy,Lisinopril, Fetal/Neonatal Morbidity and Mortality.
HydrochlorothiazideWARNINGS, Hepatic Failure WARNINGS
OVERDOSAGE
WARNINGSAnaphylactoid Reactions During Membrane Exposure
DOSAGE & ADMINISTRATION
WARNINGS
To minimize dose-independent side effects, it is usually appropriate to begin combination therapy only after a patient has failed to achieve the desired effect with monotherapy.
Dose Titration Guided by Clinical Effect
A patient whose blood pressure is not adequately controlled with either lisinopril or hydrochlorothiazide monotherapy may be switched to lisinopril/HCTZ 10/12.5 or lisinopril/HCTZ 20/12.5, depending on current monotherapy dose. Further increases of either or both components could depend on clinical response with blood pressure measured at the interdosing interval to ensure that there is an adequate antihypertensive effect at that time. The hydrochlorothiazide dose should generally not be increased until 2-3 weeks have elapsed. After addition of the diuretic it may be possible to reduce the dose of lisinopril. Patients whose blood pressures are adequately controlled with 25 mg of daily hydrochlorothiazide, but who experience significant potassium loss with this regimen, may achieve similar or greater blood-pressure control without electrolyte disturbance if they are switched to lisinopril/HCTZ 10/12.5.
In patients who are currently being treated with a diuretic, symptomatic hypotension occasionally may occur following the initial dose of lisinopril. The diuretic should, if possible, be discontinued for two to three days before beginning therapy with lisinopril to reduce the likelihood of hypotension. (SeeWARNINGS.) If the patient's blood pressure is not controlled with lisinopril alone, diuretic therapy may be resumed.
If the diuretic cannot be discontinued, an initial dose of 5 mg of lisinopril should be used under medical supervision for at least two hours and until blood pressure has stabilized for at least an additional hour. (SeeWARNINGSandPRECAUTIONS,Drug Interactions.)
Concomitant administration of lisinopril and hydrochlorothiazide with potassium supplements, potassium salt substitutes or potassium-sparing diuretics may lead to increases of serum potassium. (SeePRECAUTIONS.)
Replacement Therapy
The combination may be substituted for the titrated individual components.
Use in Renal Impairment
Regimens of therapy with lisinopril/HCTZ need not take account of renal function as long as the patient's creatinine clearance is >30 mL/min/1.7 m2 (serum creatinine roughlymg/dL or 265In patients with more severe renal impairment, loop diuretics are preferred to thiazides, so lisinopril/HCTZ is not recommended (seeWARNINGS,Anaphylactoid Reactions During Membrane Exposure).
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Lisinopril and HydrochlorothiazideLisinopril and Hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!