Doxycycline Hyclate
FULL PRESCRIBING INFORMATION: CONTENTS*
- Rx only
- DOXYCYCLINE HYCLATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- DOXYCYCLINE HYCLATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DOXYCYCLINE HYCLATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
Rx only
DOXYCYCLINE HYCLATE DESCRIPTION
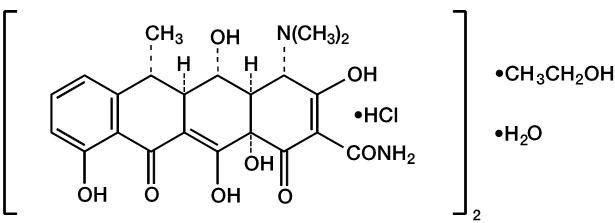
(C22H24N2O8M.W. 1025.89
CLINICAL PHARMACOLOGY
Microbiology
Gram-Negative Bacteria
Gram-Positive Bacteria
Other Microorganisms
Susceptibility tests: Diffusion techniques:Quantitative methods that require measurement of zone diameters give the most precise estimate of the susceptibility of bacteria to antimicrobial agents. One such standard procedure1 which has been recommended for use with disks to test susceptibility of organisms to doxycycline uses the 30-mcg tetracycline-class disk or the 30-mcg doxycycline disk. Interpretation involves the correlation of the diameter obtained in the disk test with the minimum inhibitory concentration (MIC) for tetracycline or doxycycline, respectively.
Zone Diameter (mm) Interpretation tetracycline doxycycline
Organism Zone Diameter (mm) tetracycline doxycycline
Dilution techniques:Use a standardized dilution method2 (broth, agar, microdilution) or equivalent with tetracycline powder. The MIC values obtained should be interpreted according to the following criteria:
MIC (mcg/mL) Interpretation
Organism MIC (mcg/mL)
INDICATIONS & USAGE
Treatment:
Prophylaxis:
DOSAGE AND ADMINISTRATIONsection and Information for Patientssubsection of the PRECAUTIONSsection.)
DOXYCYCLINE HYCLATE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralInformation for Patients
WARNINGS.)
ADVERSE REACTIONS.)
DRUG INTERACTIONS.)
DRUG INTERACTIONS.)
Laboratory Tests
Drug Interactions
Drug/Laboratory Test Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pregnancy:Teratogenic Effects. Pregnancy Category D:
Nonteratogenic Effects:(See WARNINGS.)
Labor and Delivery
Nursing Mothers
Tetracyclines are excreted in human milk; however, the extent of absorption of tetracyclines, including dpxycycline, by the breastfed infant is not known. Short-term use by lactating women is not necessarilt contraindicated; however, the effects of prolonged exposure to doxycycline in breast milk are unknown. Because of the potential for serious adverse reactions in nursing infants from doxycycline, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. (See WARNINGS.)
Pediatric Use
WARNINGSand DOSAGE AND ADMINISTRATION.
DOXYCYCLINE HYCLATE ADVERSE REACTIONS
DOSAGE AND ADMINISTRATION.)
WARNINGS.)
WARNINGS.)
PRECAUTIONSGeneral.)
OVERDOSAGE
DOSAGE & ADMINISTRATION
ADVERSE REACTIONS.)
HOW SUPPLIED
STORAGE AND HANDLING
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
REFERENCES
1.National Committee for Clinical Laboratory Standards,Performance Standards for Antimicrobial Disk Susceptibility Tests,Fourth Edition. Approved Standard NCCLS Document M2-A4, Vol. 10,No. 7 NCCLS,Villanova, PA, April 1990.2.National Committee for Clinical Laboratory Standards,Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically,Second Edition. Approved Standard NCCLS Document M7-A2,Vol. 10,No. 8 NCCLS,Villanova,PA, April 1990.
3.aFriedman JM and Polifka JE. Teratogenic Effects of Drugs. A Resource for Clinicians (TERIS). Baltimore,MD:The Johns Hopkins University Press, 2000:149-195.
cCziezel AE and Rockenbauer M. Teratogenic study of doxycycline. Obstet Gynecol 1997;89:524-528.
cHorne HW Jr and Kundsin RB. The role of mycoplasma among 81 consecutive pregnancies:a prospective study. Int J Fertil 1980;25:315-317.
dHale T. Medications and Mothers Milk. 9th edition. Amarillo, TX: Pharmasoft Publishing,2000:225
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Doxycycline HyclateDoxycycline Hyclate CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!