Acetaminophen and Codeine
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- ACETAMINOPHEN AND CODEINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ACETAMINOPHEN AND CODEINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR OWNERS/CAREGIVERS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- ACETAMINOPHEN AND CODEINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Hepatotoxicity:Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product.
ACETAMINOPHEN AND CODEINE DESCRIPTION
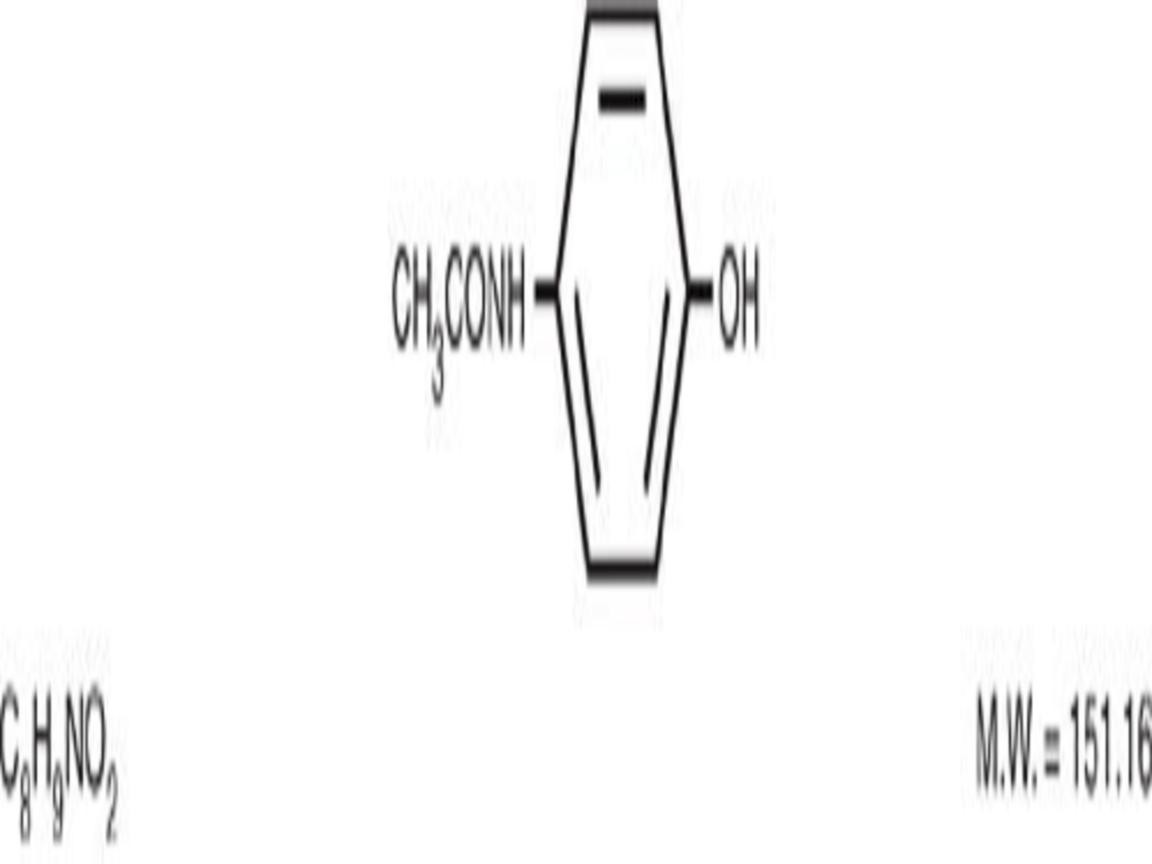
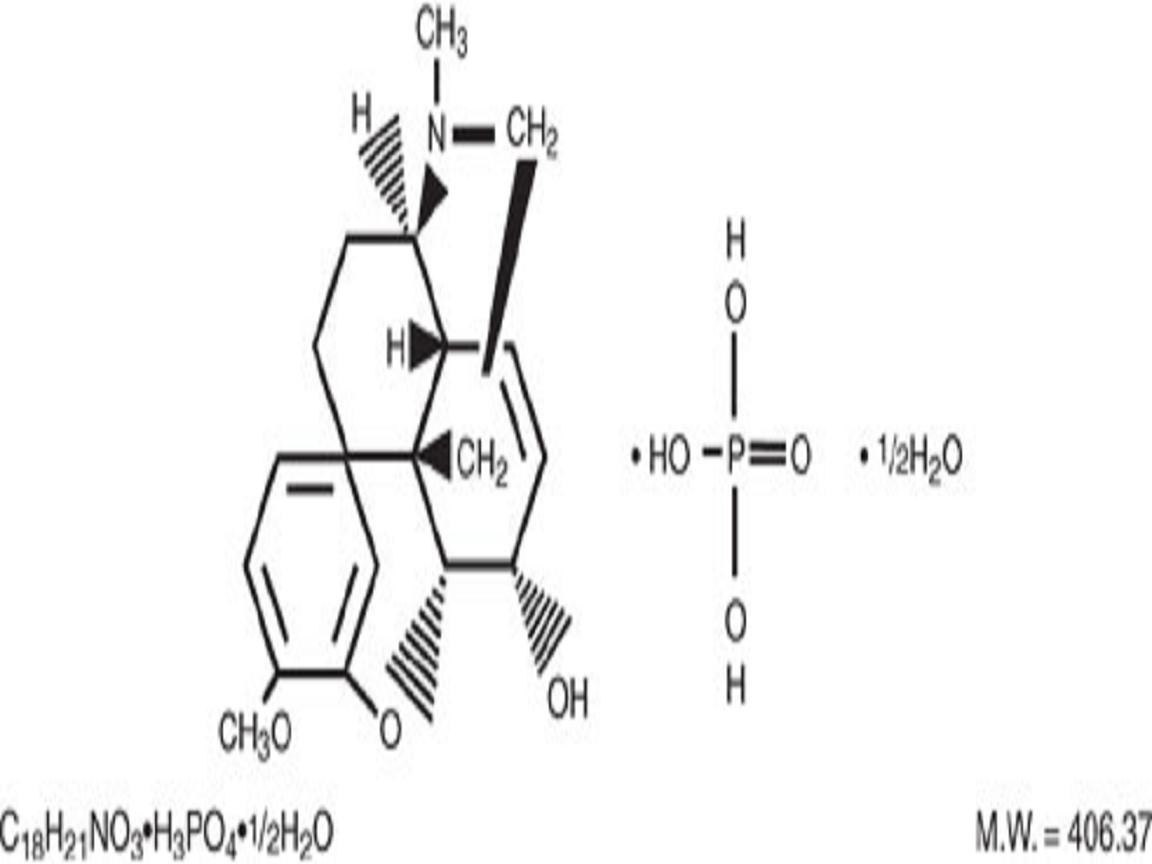
CLINICAL PHARMACOLOGY
Pharmacokinetics:
Codeine:
OVERDOSAGE
Acetaminophen:
OVERDOSAGE
INDICATIONS & USAGE
ACETAMINOPHEN AND CODEINE CONTRAINDICATIONS
WARNINGS
Hepatotoxicity:Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
Hypersensitivity/anaphylaxis:
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue Acetaminophen and Codeine Phosphate Tablets, USP immediately and seek medical care if they experience these symptoms. Do not prescribe Acetaminophen and Codeine Phosphate Tablets, USP for patients with acetaminophen allergy.
PRECAUTIONS
General:Ultra-Rapid Metabolizers of Codeine
PRECAUTIONS - Nursing Mothers
INFORMATION FOR OWNERS/CAREGIVERS
-
● Do not take Acetaminophen and Codeine Phosphate Tablets, USP if you are allergic to any of its ingredients.
-
● If you develop signs of allergy such as a rash or difficulty breathing stop taking Acetaminophen and Codeine Phosphate Tablets, USP and contact your healthcare provider immediately.
-
● Do not take more than 4000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose.
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects: Pregnancy Category C:Nonteratogenic Effects:
LABOR & DELIVERY
OVERDOSAGENURSING MOTHERS
PRECAUTIONS - General - Ultra-Rapid Metabolizers of Codeine
ACETAMINOPHEN AND CODEINE ADVERSE REACTIONS
DRUG ABUSE AND DEPENDENCE
Controlled Substance:Abuse and Dependence:
OVERDOSAGE
Signs and Symptoms:
Treatment:
DOSAGE & ADMINISTRATION
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Acetaminophen and CodeineAcetaminophen and Codeine TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!