Zofran
FULL PRESCRIBING INFORMATION: CONTENTS*
- ZOFRAN DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- ZOFRAN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ZOFRAN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ZOFRAN DESCRIPTION
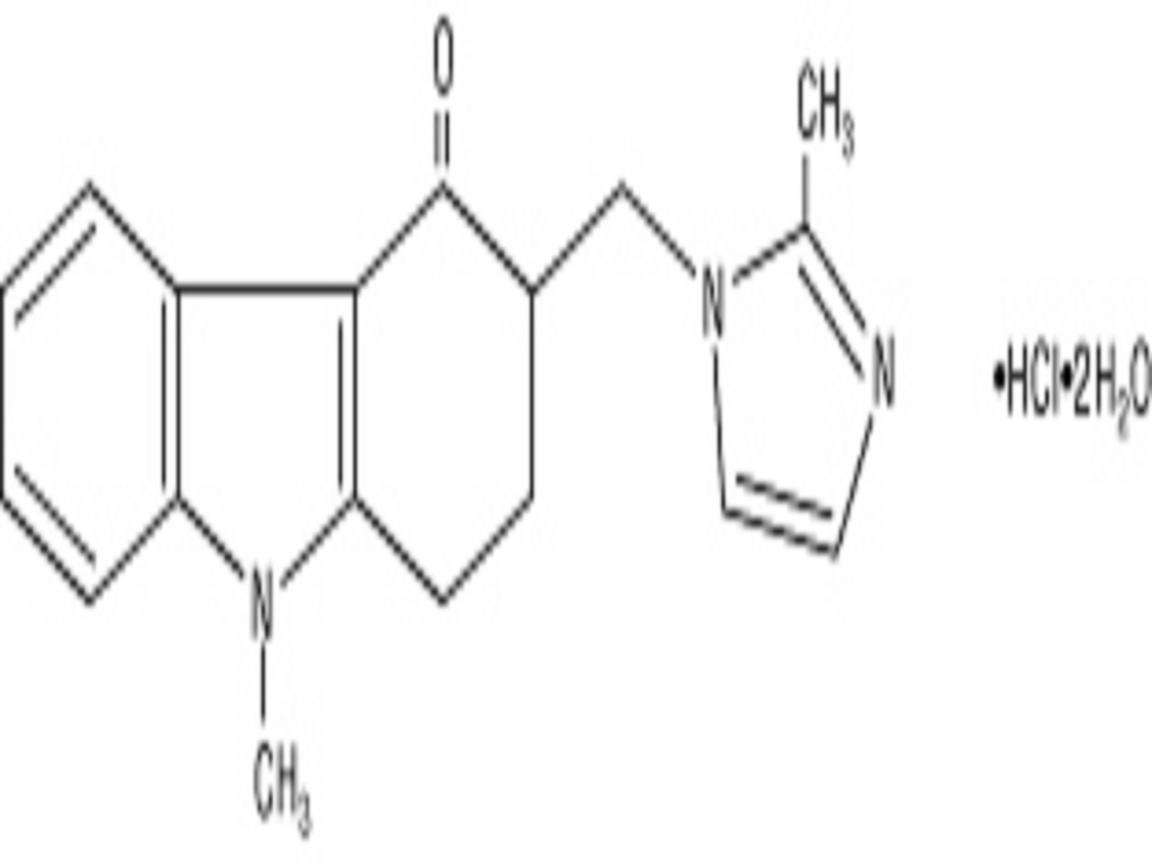
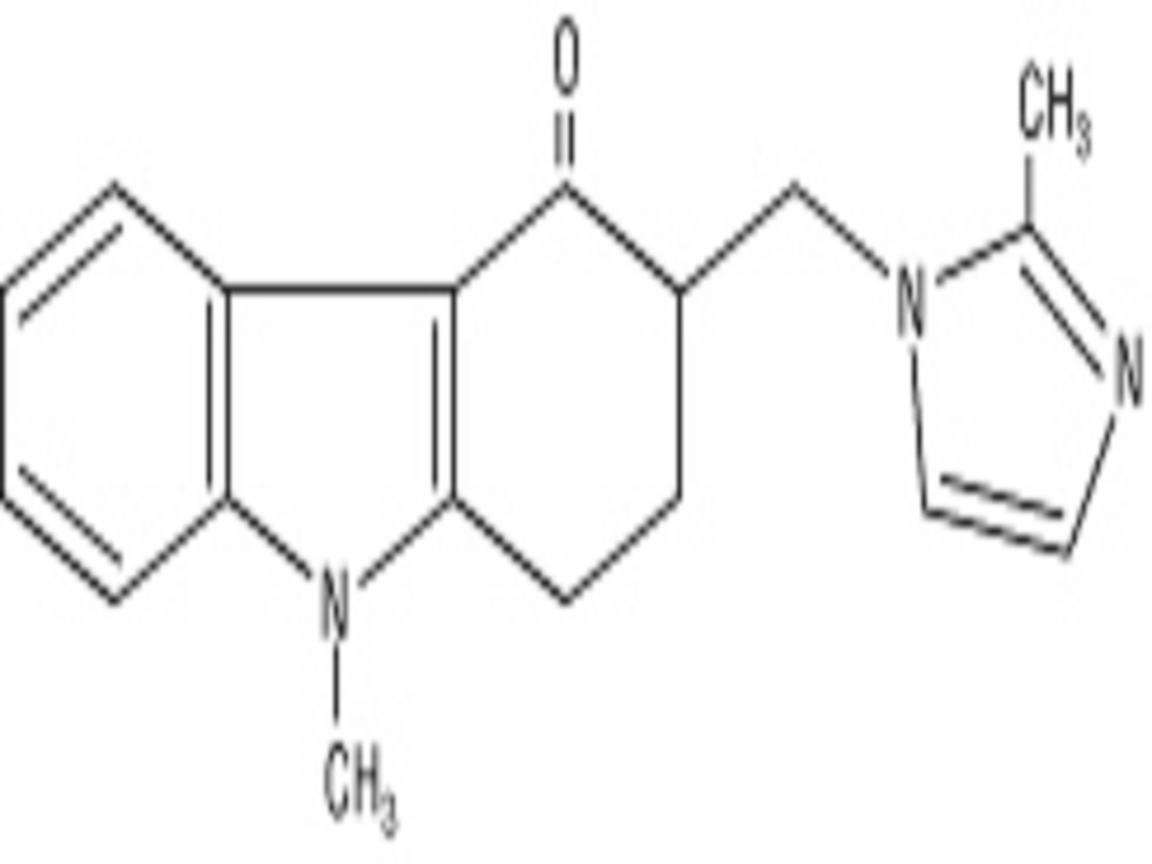
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
CLINICAL TRIALS
Chemotherapy-Induced Nausea and Vomiting
Highly Emetogenic Chemotherapy
Moderately Emetogenic Chemotherapy
Re-treatment
Pediatric Studies
Radiation-Induced Nausea and Vomiting
Total Body Irradiation
Single High-Dose Fraction Radiotherapy
Daily Fractionated Radiotherapy
Postoperative Nausea and Vomiting
INDICATIONS & USAGE
ZOFRAN CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
PhenylketonuricsDRUG INTERACTIONS
Apomorphine
Phenytoin, Carbamazepine, and Rifampicin
Tramadol
Chemotherapy
Use in Surgical Patients
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsNURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
ZOFRAN ADVERSE REACTIONS
Chemotherapy-Induced Nausea and Vomiting
Central Nervous System
Hepatic
Integumentary
Other
Radiation-Induced Nausea and Vomiting
Postoperative Nausea and Vomiting
Observed During Clinical Practice
Cardiovascular
General
Hepatobiliary
Lower Respiratory
Neurology:
Skin
Special Senses
Eye Disorders
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
Instructions for Use/Handling ZOFRAN ODT Orally Disintegrating TabletsPrevention of Nausea and Vomiting Associated With Highly Emetogenic Cancer Chemotherapy
Pediatric Use
Geriatric Use
Prevention of Nausea and Vomiting Associated With Moderately Emetogenic Cancer Chemotherapy
Pediatric Use
Geriatric Use
Prevention of Nausea and Vomiting Associated With Radiotherapy, Either Total Body Irradiation, or Single High-Dose Fraction or Daily Fractions to the Abdomen
Pediatric Use
Geriatric Use
Postoperative Nausea and Vomiting
Pediatric Use
Geriatric Use
Dosage Adjustment for Patients With Impaired Renal Function
Dosage Adjustment for Patients With Impaired Hepatic Function
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

ZofranOndansetron Hydrochloride TABLET, ORALLY DISINTEGRATING
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!