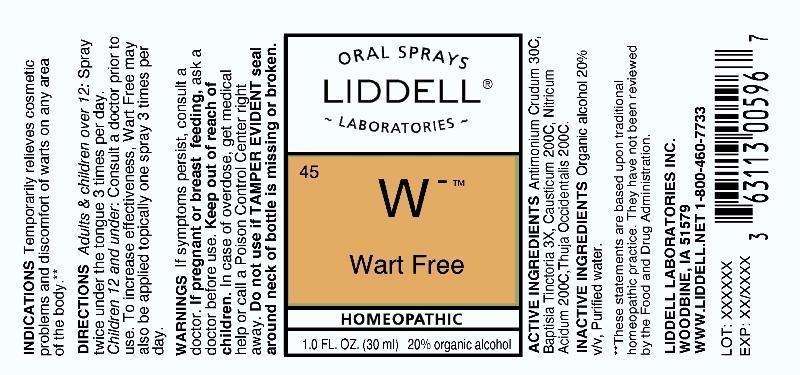
Wart Free
Liddell Laboratories, Inc.
Liddell Laboratories, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
ACTIVE INGREDIENTS: Antimonium crudum 30C, Baptisia tinctoria 3X, Causticum 200C, Nitricum acidum 200C, Thuja occidentalis 200C.
Purpose
INDICATIONS: Temporarily relieves cosmetic problems and discomfort of warts on any area of the body.
WARNINGS: If symptoms persist, consult a doctor.
If pregnant or breast feeding, ask a doctor before use.
Keep out of reach of children. In case of overdose, get medical help or call a Poison Control Center right away.
Do not use if TAMPER EVIDENT seal around neck of bottle is missing or broken.
DIRECTIONS: Adults and children over 12: Spray twice under the tongue 3 times per day. Children 12 and under: Consult a doctor prior to use.
Wart Free can also be applied topically to increase effectiveness.
INACTIVE INGREDIENTS: Organic alcohol 20% v/v, Purified water.
KEEP OUT OF REACH OF CHILDREN. In case of overdose, get medical help or call a Poison Control Center right away.
Uses
INDICATIONS: Temporarily relieves cosmetic problems and discomfort of warts on any area of the body.
LIDDELL LABORATORIES
WOODBINE, IA 51579
WWW.LIDDELL.NET
1-800-460-7733
ORAL SPRAYS
LIDDELL LABORATORIES
45
W
Wart Free
HOMEOPATHIC
1.0 FL. OZ. (30 ml)
Wart FreeAntimonium crudum, Baptisia tinctoria, Causticum, Nitricum acidum, Thuja occidentalis, SPRAY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||