Visible Difference Gentle Hydrating Broad Spectrum Sunscreen SPF 15
BC1169
FULL PRESCRIBING INFORMATION: CONTENTS*
- VISIBLE DIFFERENCE GENTLE HYDRATING BROAD SPECTRUM SUNSCREEN SPF 15 DESCRIPTION
- VISIBLE DIFFERENCE GENTLE HYDRATING BROAD SPECTRUM SUNSCREEN SPF 15 INDICATIONS AND USAGE
- WARNINGS
- OTC - ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- VISIBLE DIFFERENCE GENTLE HYDRATING BROAD SPECTRUM SUNSCREEN SPF 15 DOSAGE AND ADMINISTRATION
- OTC - KEEP OUT OF REACH OF CHILDREN
- OTC - PURPOSE
- WHEN USING
FULL PRESCRIBING INFORMATION
VISIBLE DIFFERENCE GENTLE HYDRATING BROAD SPECTRUM SUNSCREEN SPF 15 DESCRIPTION
This multi-benefit moisturizer for dry skin provides intense hydration. Leaves skin feeling soft and smooth.
VISIBLE DIFFERENCE GENTLE HYDRATING BROAD SPECTRUM SUNSCREEN SPF 15 INDICATIONS AND USAGE
To Use: Smooth onto cleansed face and throat.
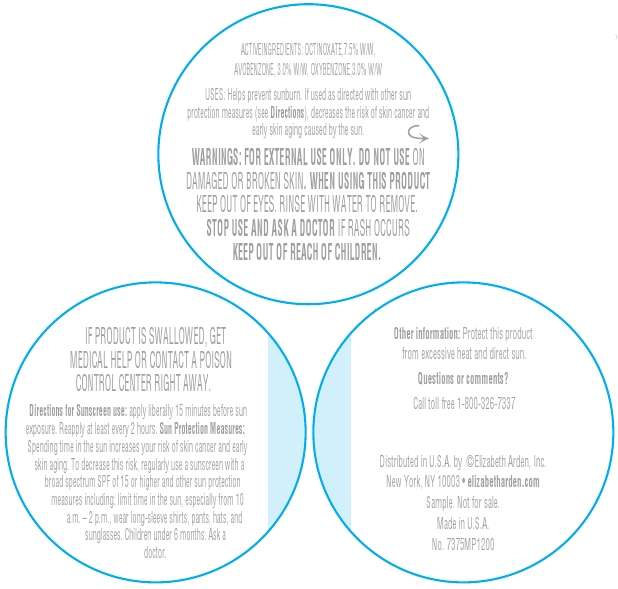
Directions For Sunscreen Use: Apply liberally 15 minutes before sun exposure. Reapply at least every 2 hours. Sun Protection Measures. Spending time in the sun increases your risk of measures including: Limit time in the sun, especially from 10 a.m. – 2 p.m. Wear long-sleeve shirts, pants, decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection hats, and sunglasses.
Children under 6 months ask a doctor.
WARNINGS
Warnings: For external use only. Do not use on damaged or broken skin. When using this product keep out of eyes. Rinse with water to remove. Stop use and ask a doctor if rash occurs.
OTC - ACTIVE INGREDIENT
Active Ingredients: Octinoxate 7.5%, Avobenzone 3.0%, Oxybenzone 3.0%.
INACTIVE INGREDIENT
Inactive Ingredients: Water/Aqua/Eau, Butylene Glycol, Glycerin, Isononyl Isononanoate, Dimethicone, Cetearyl Glucoside, Pentylene Glycol, Butyl Stearate, Tridecyl Salicylate, Cetyl Ricinoleate, Cyclopentasiloxane, Narcissus Tazetta Bulb Extrat, Trifolium Pratense (Clover) Flower Extract, Sodium Hyaluronate, Tocopherol, Tocopheryl Acetate, Caprylyl Glycol, Cetearyl Dimethicone Crosspolymer, Ethylhexyl Palmitate, Glyceryl Stearate, Sodium PCA, Trehalose, Urea, Hyrdolyzed Soy Protein, Lecithin, Phospholipids, Polyphophorylcholine Glycol Acrylate, Ammonium Acryloyldimethylaurate/VP Coplymer, PEG-100 Stearate, PEG-8 Laurate, PEG-60 Hydrogenated Castor Oil, Sodium Dodecylbenzenesulfonate, Polyquaternium-51, Styrene/Acrylates Copolymer, Hexylene Glycol, Isododecane, PEG-8, Xanthan Gum, Nylon-12, BHT, Disodium EDTA, Mica, Tin Oxide, Silica Dimethyl Silylate, Parfum/Fragrance, Benzyl Salicylate, Butylphenyl Methylpropional, Citonellol, Hydroxyisohexyl 3-Cyclohexene Carboxaldehyde, Limnene, Linalool, Benzoic Acid, Butylparaben, Ethylparaben, Isobutylparaben, Methylparaben, Phenoxyethanol, Potassium Sorbate, Propylparaben, Sorbic Acid, Chlorphenesin, Titanium Dioxide (CI 77891).
VISIBLE DIFFERENCE GENTLE HYDRATING BROAD SPECTRUM SUNSCREEN SPF 15 DOSAGE AND ADMINISTRATION
Smooth on to face and throat.
OTC - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
OTC - PURPOSE
Provides SPF 15 Sun Protection.
WHEN USING
Keep out of eyes.