Vincasar PFS
Teva Parenteral Medicines, Inc.
Vincasar PFS (vinCRIStine sulfate Injection, USP)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNINGS
- VINCASAR PFS DESCRIPTION
- CLINICAL PHARMACOLOGY
- VINCASAR PFS INDICATIONS AND USAGE
- VINCASAR PFS CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- VINCASAR PFS ADVERSE REACTIONS
- OVERDOSAGE
- VINCASAR PFS DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Package Insert
Rx only
4402
4412
PRESERVATIVE FREE SOLUTION
WARNINGS
Caution-This preparation should be administered by individuals experienced in the administration of VINCASAR PFS. It is extremely important that the intravenous needle or catheter be properly positioned before any vincristine is injected. Leakage into surrounding tissue during intravenous administration of VINCASAR PFS may cause considerable tissue irritation. If extravasation occurs, the injection should be discontinued immediately, and any remaining portion of the dose should then be introduced into another vein. Local injection of hyaluronidase and the application of moderate heat to the area of leakage help disperse the drug and are thought to minimize discomfort and the possibility of cellulitis.
FOR INTRAVENOUS USE ONLY – FATAL IF GIVEN BY OTHER ROUTES.
See OVERDOSAGE section for the treatment of patients given intrathecal VINCASAR PFS.
VINCASAR PFS DESCRIPTION
VINCASAR PFS (vincristine sulfate injection, USP) is vincaleukoblastine, 22-oxo-, sulfate (1:1) (salt). It is the salt of an alkaloid obtained from a common flowering herb, the periwinkle plant (Vinca rosea Linn). Originally known as leurocristine, it has also been referred to as LCR and VCR. The structural formula is as follows:
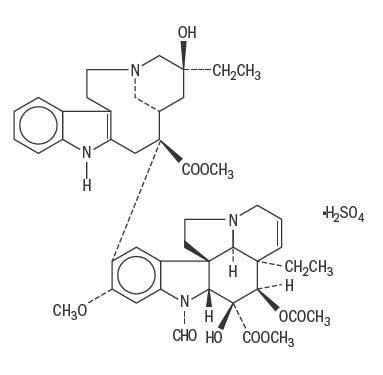
C46H56N4O10•H2SO4 M.W. 923.04
Vincristine Sulfate, USP is a white to off-white powder. It is soluble in methanol, freely soluble in water, but only slightly soluble in 95% ethanol. In 98% ethanol, vincristine sulfate, USP has an ultraviolet spectrum with maxima at 221 nm (∈+47,100).
Vincristine Sulfate Injection USP is a sterile, preservative-free, single use only solution available for intravenous use in 1 mg (1 mg/1 mL) and 2 mg (2 mg/2 mL) vials. Each mL contains vincristine sulfate, USP, 1 mg (1.08 µmol); mannitol, 100 mg; and water for injection USP, qs. Acetic acid and sodium acetate have been added for pH control. The pH of VINCASAR PFS ranges from 3.5 to 5.5. This product is a sterile solution for cancer/oncolytic use.
CLINICAL PHARMACOLOGY
The mechanisms of action of vincristine sulfate remain under investigation. The mechanism of action of vincristine sulfate has been related to the inhibition of microtubule formation in mitotic spindle, resulting in an arrest of dividing cells at the metaphase stage.
Central nervous system leukemia has been reported in patients undergoing otherwise successful therapy with vincristine sulfate. This suggests that vincristine does not penetrate well into the cerebrospinal fluid.
Pharmacokinetic studies in patients with cancer have shown a triphasic serum decay pattern following rapid intravenous injection. The initial, middle, and terminal half-lives are 5 minutes, 2.3 hours, and 85 hours respectively; however, the range of the terminal half-life in humans is from 19 to 155 hours. The liver is the major excretory organ in humans and animals. The metabolism of vinca alkaloids has been shown to be mediated by hepatic cytochrome P450 isoenzymes in the CYP 3A subfamily. This metabolic pathway may be impaired in patients with hepatic dysfunction or who are taking concomitant potent inhibitors of these isoenzymes (see PRECAUTIONS ). About 80% of an injected dose of vincristine sulfate appears in the feces and 10% to 20% can be found in the urine. Within 15 to 30 minutes after injection, over 90% of the drug is distributed from the blood into tissue, where it remains tightly, but not irreversibly, bound.
Current principles of cancer chemotherapy involve the simultaneous use of several agents. Generally, each agent used has a unique toxicity and mechanism of action so that therapeutic enhancement occurs without additive toxicity. It is rarely possible to achieve equally good results with single-agent methods of treatment. Thus, vincristine sulfate is often chosen as part of polychemotherapy because of lack of significant bone-marrow suppression (at recommended doses) and of unique clinical toxicity (neuropathy). See DOSAGE AND ADMINISTRATION section for possible increased toxicity when used in combination therapy.
VINCASAR PFS INDICATIONS AND USAGE
VINCASAR PFS is indicated in acute leukemia.
VINCASAR PFS has also been shown to be useful in combination with other oncolytic agents in Hodgkin’s disease, non-Hodgkin’s malignant lymphomas, rhabdomyosarcoma, neuroblastoma, and Wilms’ tumor.
VINCASAR PFS CONTRAINDICATIONS
Patients with the demyelinating form of Charcot-Marie-Tooth syndrome should not be given VINCASAR PFS. Careful attention should be given to those conditions listed under WARNINGS and PRECAUTIONS .
WARNINGS
WARNINGS
This preparation is for intravenous use only. It should be administered by individuals experienced in the administration of vincristine sulfate injection. The intrathecal administration of VINCASAR PFS usually results in death.
To reduce the potential for fatal medication errors due to incorrect route of administration, VINCASAR PFS should be diluted in a flexible plastic container and prominently labeled as indicated for intravenous use only.
Syringes containing this product must be labeled, using auxiliary sticker provided, to state “FOR INTRAVENOUS USE ONLY – FATAL IF GIVEN BY OTHER ROUTE.”
Extemporaneously prepared syringes containing this product must be packaged in an overwrap which is labeled “do not remove covering until moment of injection. For intravenous use only – fatal if given by other routes.”
See OVERDOSAGE section for the treatment of patients given intrathecal VINCASAR PFS.
Pregnancy Category D
Vincristine sulfate can cause fetal harm when administered to a pregnant woman. When pregnant mice and hamsters were given doses of vincristine sulfate that caused resorption of 23% to 85% of fetuses, fetal malformations were produced in those that survived. Five monkeys were given single doses of vincristine sulfate between days 27 and 34 of their pregnancies; 3 of the fetuses were normal at term, and 2 viable fetuses had grossly evident malformations at term . In several animal species, vincristine sulfate can induce teratogenesis as well as embryo death at doses that are nontoxic to the pregnant animal. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or if the patient becomes pregnant while receiving this drug, she should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
PRECAUTIONS
General
Acute uric acid nephropathy, which may occur after the administration of oncolytic agents, has also been reported with vincristine sulfate. In the presence of leukopenia or a complicating infection, administration of the next dose of VINCASAR PFS warrants careful consideration.
If central nervous system leukemia is diagnosed, additional agents may be required, because vincristine does not appear to cross the blood-brain barrier in adequate amounts.
Particular attention should be given to dosage and neurologic side effects if VINCASAR PFS is administered to patients with preexisting neuromuscular disease and when other drugs with neurotoxic potential are also being used.
Acute shortness of breath and severe bronchospasm have been reported following the administration of vinca alkaloids. These reactions have been encountered most frequently when the vinca alkaloid was used in combination with mitomycin-C and may require aggressive treatment, particularly when there is preexisting pulmonary dysfunction. The onset of these reactions may occur minutes to several hours after the vinca alkaloid is injected and may occur up to 2 weeks following the dose of mitomycin. Progressive dyspnea requiring chronic therapy may occur. VINCASAR PFS should not be readministered.
Care must be taken to avoid contamination of the eye with concentration of VINCASAR PFS used clinically. If accidental contamination occurs, severe irritation (or, if the drug was delivered under pressure, even corneal ulceration) may result. The eye should be washed immediately and thoroughly.
Laboratory Tests
Because dose-limiting clinical toxicity is manifested as neurotoxicity, clinical evaluation (e.g., history, physical examination) is necessary to detect the need for dosage modification. Following administration of VINCASAR PFS, some individuals may have a fall in the white blood cell count or platelet count, particularly when previous therapy or the disease itself has reduced bone-marrow function. Therefore, a complete blood count should be done before administration of each dose. Acute elevation of serum uric acid may also occur during induction of remission in acute leukemia; thus, such levels should be determined frequently during the first 3 to 4 weeks of treatment or appropriate measures taken to prevent uric acid nephropathy. The laboratory performing these tests should be consulted for its range of normal values.
Drug Interactions
The simultaneous oral or intravenous administration of phenytoin and antineoplastic chemotherapy combinations that included vincristine sulfate has been reported to reduce blood levels of the anticonvulsant and to increase seizure activity. Dosage adjustment should be based on serial blood level monitoring. The contribution of vincristine sulfate to this interaction is not certain. The interaction may result from reduced absorption of phenytoin and an increase in the rate of its metabolism and elimination.
Caution should be exercised in patients concurrently taking drugs known to inhibit drug metabolism by hepatic cytochrome P450 isoenzymes in the CYP 3A subfamily, or in patients with hepatic dysfunction. Concurrent administration of vincristine sulfate with itraconazole (a known inhibitor of the metabolic pathway) has been reported to cause an earlier onset and/or an increased severity of neuromuscular side effects (see ADVERSE REACTIONS ). This interaction is presumed to be related to inhibition of the metabolism of vincristine.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Neither in vivo nor in vitro laboratory tests have conclusively demonstrated the mutagenicity of this product. Fertility following treatment with vincristine sulfate alone for malignant disease has not been studied in humans. Clinical reports of both male and female patients who received multiple-agent chemotherapy that included vincristine sulfate indicate that azoospermia and amenorrhea can occur in postpubertal patients. Recovery occurred many months after completion of chemotherapy in some but not all patients. When the same treatment is administered to prepubertal patients, permanent azoospermia and amenorrhea are much less likely.
Patients who received chemotherapy with vincristine sulfate in combination with anticancer drugs known to be carcinogenic have developed second malignancies. The contributing role of vincristine sulfate in this development has not been determined. No evidence of carcinogenicity was found following intraperitoneal administration of vincristine sulfate in rats and mice, although this study was limited.
USAGE IN PREGNANCY
Teratogenic Effects
Pregnancy Category D
See WARNINGS.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions due to vincristine sulfate in nursing infants, a decision should be made either to discontinue nursing or the drug, taking into account the importance of the drug to the mother.
Pediatric Use
See DOSAGE AND ADMINISTRATION section.
VINCASAR PFS ADVERSE REACTIONS
In general, adverse reactions are reversible and are related to dosage. The most common adverse reaction is hair loss; the most troublesome adverse reactions are neuromuscular in origin.
When single, weekly doses of the drug are employed, the adverse reactions of leukopenia, neuritic pain, and constipation occur but are usually of short duration (i.e., less than 7 days). When the dosage is reduced, these reactions may lessen or disappear. The severity of such reactions seems to increase when the calculated amount of drug is given in divided doses. Other adverse reactions, such as hair loss, sensory loss, paresthesia, difficulty in walking, slapping gait, loss of deep-tendon reflexes, and muscle wasting, may persist for at least as long as therapy is continued. Generalized sensorimotor dysfunction may become progressively more severe with continued treatment. Although most such symptoms usually disappear by about the sixth week after discontinuance of treatment, some neuromuscular difficulties may persist for prolonged periods in some patients. Regrowth of hair may occur while maintenance therapy walking, slapping gait, loss of deep-tendon reflexes, and muscle wasting, may persist for at least as long as therapy is continued. Generalized sensorimotor dysfunction may become progressively more severe with continued treatment. Although most such symptoms usually disappear by about the sixth week after discontinuance of treatment, some neuromuscular difficulties may persist for prolonged periods in some patients. Regrowth of hair may occur while maintenance therapy continues.
The following adverse reactions have been reported:
Hepatic veno-occlusive disease has been reported in patients receiving vincristine, particularly in pediatric patients, as part of standard combination chemotherapy regimens. Some of the patients had fatal outcomes; some who survived had undergone liver transplantation.
Hypersensitivity
Rare cases of allergic-type reactions, such as anaphylaxis, rash, and edema, that are temporally related to vincristine therapy have been reported in patients receiving vincristine as a part of multidrug chemotherapy regimens.
Gastrointestinal
Constipation, abdominal cramps, weight loss, nausea, vomiting, oral ulceration, diarrhea, paralytic ileus, intestinal necrosis and/or perforation, and anorexia have occurred. Constipation may take the form of upper-colon impaction, and, on physical examination, the rectum may be empty. Colicky abdominal pain coupled with an empty rectum may mislead the physician. A flat film of the abdomen is useful in demonstrating this condition. All cases have responded to high enemas and laxatives. A routine prophylactic regimen against constipation is recommended for all patients receiving VINCASAR PFS.
Paralytic ileus (which mimics the "surgical abdomen") may occur, particularly in young pediatric patients. The ileus will reverse itself with temporary discontinuance of VINCASAR PFS injection and with symptomatic care.
Genitourinary
Polyuria, dysuria, and urinary retention due to bladder atony have occurred. Other drugs known to cause urinary retention (particularly in the elderly) should, if possible, be discontinued for the first few days following administration of VINCASAR PFS.
Cardiovascular
Hypertension and hypotension have occurred. Chemotherapy combinations that have included vincristine sulfate, when given to patients previously treated with mediastinal radiation, have been associated with coronary artery disease and myocardial infarction. Causality has not been established.
Neurologic
Frequently, there is a sequence to the development of neuromuscular side effects. Initially, only sensory impairment and paresthesia may be encountered. With continued treatment, neuritic pain and, later, motor difficulties may occur. There have been no reports of any agent that can reverse the neuromuscular manifestations that may accompany therapy with vincristine sulfate.
Loss of deep-tendon reflexes, foot drop, ataxia, and paralysis have been reported with continued administration. Cranial nerve manifestations, such as isolated paresis and/or paralysis of muscles controlled by cranial motor nerves including potentially life-threatening bilateral vocal cord paralysis, may occur in the absence of motor impairment elsewhere; extraocular and laryngeal muscles are those most commonly involved. Jaw pain, pharyngeal pain, parotid gland pain, bone pain, back pain, limb pain, and myalgias have been reported; pain in these areas may be severe. Convulsions, frequently with hypertension, have been reported in a few patients receiving vincristine sulfate. Several instances of convulsions followed by coma have been reported in pediatric patients. Transient cortical blindness and optic atrophy with blindness have been reported. Treatment with vinca alkaloids has resulted in both vestibular and auditory damage to the eighth cranial nerve. Manifestations include partial or total deafness which may be temporary or permanent, and difficulties with balance including dizziness, nystagmus, and vertigo. Particular caution is warranted when vincristine is used in combination with other agents known to be ototoxic such as the platinum-containing oncolytics.
Pulmonary
See PRECAUTIONS.
Endocrine
Rare occurrences of a syndrome attributable to inappropriate antidiuretic hormone secretion have been observed in patients treated with vincristine sulfate. This syndrome is characterized by high urinary sodium excretion in the presence of hyponatremia; renal or adrenal disease, hypotension, dehydration, azotemia, and clinical edema are absent. With fluid deprivation, improvement occurs in the hyponatremia and in the renal loss of sodium.
Hematologic
VINCASAR PFS does not appear to have any constant or significant effect on platelets or red blood cells. Serious bone-marrow depression is usually not a major dose-limiting event. However, anemia, leukopenia, and thrombocytopenia have been reported. Thrombocytopenia, if present when therapy with VINCASAR PFS is begun, may actually improve before the appearance of bone marrow remission.
Skin
Alopecia and rash have been reported.
Other
Fever and headache have occurred.
OVERDOSAGE
Side effects following the use of VINCASAR PFS are dose related. In pediatric patients under 13 years of age, death has occurred following doses of vincristine sulfate that were 10 times those recommended for therapy. Severe symptoms may occur in this patient group following dosages of 3 to 4 mg/m2. Adults can be expected to experience severe symptoms after single doses of 3 mg/m2 or more (see ADVERSE REACTIONS ). Therefore, following administration of doses higher than those recommended, patients can be expected to experience exaggerated side effects. Supportive care should include the following: (1) prevention of side effects resulting from the syndrome of inappropriate antidiuretic hormone secretion (preventive treatment would include restriction of fluid intake and perhaps the administration of a diuretic affecting the function of Henle’s loop and the distal tubule); (2) administration of anticonvulsants; (3) use of enemas or cathartics to prevent ileus (in some instances, decompression of the gastrointestinal tract may be necessary); (4) monitoring the cardiovascular system; (5) determining daily blood counts for guidance in transfusion requirements.
Folinic acid has been observed to have a protective effect in normal mice that were administered lethal doses of vincristine sulfate (Cancer Res 1963;23:1390). Isolated case reports suggest that folinic acid may be helpful in treating humans who have received an overdose of vincristine sulfate. It is suggested that 100 mg of folinic acid be administered intravenously every 3 hours for 24 hours and then every 6 hours for at least 48 hours. Theoretically (based on pharmacokinetic data), tissue levels of vincristine sulfate can be expected to remain significantly elevated for at least 72 hours. Treatment with folinic acid does not eliminate the need for the above-mentioned supportive measures.
Most of an intravenous dose of vincristine is excreted into the bile after rapid tissue binding (see CLINICAL PHARMACOLOGY ). Because only very small amounts of the drug appear in dialysate, hemodialysis is not likely to be helpful in cases of overdosage. An increase in the severity of side effects may be experienced by patients with liver disease that is severe enough to decrease biliary excretion.
Enhanced fecal excretion of parenterally administered vincristine has been demonstrated in dogs pretreated with cholestyramine. There are no published clinical data on the use of cholestyramine as an antidote in humans.
There are no published clinical data on the consequences of oral ingestion of vincristine. Should oral ingestion occur, the stomach should be evacuated. Evacuation should be followed by oral administration of activated charcoal and a cathartic.
Treatment of patients following intathecal administration of vincristine sulfate injection has included immediate removal of spinal fluid and flushing with Lactated Ringer’s, as well as other solutions and has not prevented ascending paralysis and death. In one case, progressive paralysis in an adult was arrested by the following treatment initiated immediately after the intrathecal injection:
- As much spinal fluid was removed as could be safely done through lumbar access.
- The subarachnoid space was flushed with Lactated Ringer’s solution infused continuously through a catheter in a cerebral lateral ventricle at the rate of 150 mL/h. The fluid was removed through a lumbar access.
- As soon as fresh frozen plasma became available, the frozen plasma, 25 mL, diluted in 1 L of Lactated Ringer’s solution was infused through the cerebral ventricular catheter at the rate of 75 mL/h with removal through the lumbar access. The rate of infusion was adjusted to maintain a protein level in the spinal fluid of 150 mg/dL.
- Glutamic acid, 10 g, was given intravenously over 24 hours followed by 500 mg 3 times daily by mouth for 1 mouth or until neurological dysfunction stabilized. The role of glutamic acid in this treatment is not certain and may not be essential.
VINCASAR PFS DOSAGE AND ADMINISTRATION
This preparation is for intravenous use only (see WARNINGS ).
Neurotoxicity appears to be dose related. Extreme care must be used in calculating and administering the dose of VINCASAR PFS, since overdosage may have a very serious or fatal outcome.
The usual dose of VINCASAR PFS for pediatric patients is 1.5 mg/m2 to 2 mg/m2. For pediatric patients weighing 10 kg or less, the starting dose should be 0.05 mg/kg, administered once a week. The usual dose of VINCASAR PFS for adults is 1.4 mg/m2. A 50% reduction in the dose of VINCASAR PFS is recommended for patients having a serum direct bilirubin value above 3 mg/100 mL.
The drug is administered intravenously at weekly intervals.
TO REDUCE THE POTENTIAL FOR FATAL MEDICATION ERRORS DUE TO INCORRECT ROUTE OF ADMINISTRATION, VINCASAR PFS SHOULD BE DILUTED IN A FLEXIBLE PLASTIC CONTAINER AND PROMINENTLY LABELED FOR INTRAVENOUS USE ONLY (see WARNING).
The concentration of VINCASAR PFS is 1 mg/mL. Do not add extra fluid to the vial prior to removal of the dose. Withdraw the solution of VINCASAR PFS into an accurate dry syringe, measuring the dose carefully. Do not add extra fluid to the vial in an attempt to empty it completely.
Preparation for flexible plastic container
VINCASAR PFS when diluted with 0.9% sodium chloride injection in concentrations from 0.0015 mg/mL to 0.08 mg/mL is stable for up to 24 hours when protected from light or 8 hours under normal light at 25°C.
Preparation for syringe
Special Dispensing Information
When dispensing VINCASAR PFS in a syringe, it is imperative that it be packaged in the provided overwrap which bears the following statement: “DO NOT REMOVE COVERING UNTIL MOMENT OF INJECTION. FOR INTRAVENOUS USE ONLY – FATAL IF GIVEN BY OTHER ROUTES” (see WARNINGS). A syringe containing a specific dose must be labeled, using the auxiliary sticker provided, to state: “FOR INTRAVENOUS USE ONLY – FATAL IF GIVEN BY OTHER ROUTES.”
Caution: It is extremely important that the intravenous needle or catheter be properly positioned before any vincristine is injected. Leakage into surrounding tissue during intravenous administration of VINCASAR PFS may cause considerable irritation. If extravasation occurs, the injection should be discontinued immediately and any remaining portion of the dose should then be introduced into another vein. Local injection of hyaluronidase and the application of moderate heat to the area of leakage will help disperse the drug and may minimize discomfort and the possibility of cellulitis.
VINCASAR PFS must be administered via an intact, free-flowing intravenous needle or catheter. Care should be taken that there is no leakage or swelling occurring during administration (see boxed WARNINGS ).
The solution may be injected either directly into a vein or into the tubing of a running intravenous infusion (see Drug Interactions below). Injection of VINCASAR PFS should be accomplished within 1 minute.
Patients Receiving Radiation Therapy
VINCASAR PFS should not be given to patients while they are receiving radiation therapy through radiation ports that include the liver. When VINCASAR PFS is used in combination with L-asparaginase, VINCASAR PFS should be given 12 to 24 hours before administration of the enzyme in order to minimize toxicity; administering L-asparaginase before VINCASAR PFS may reduce hepatic clearance of vincristine.
Drug Interactions
VINCASAR PFS should not be diluted in solutions that raise or lower the pH outside the range of 3.5 to 5.5. It should not be mixed with anything other than normal saline or glucose in water.
Whenever solution and container permit, parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Handling and Disposal
Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on this subject have been published.1 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
HOW SUPPLIED
VINCASAR PFS (vincristine sulfate injection USP), preservative free and is supplied as follows:
| NDC Number | VINCASAR PFS (vincristine sulfate injection, USP) | Volume |
| 0703-4402-11 | 1 mg/mL | 1 mL |
| 0703-4412-11 | 1 mg/mL | 2 mL |
This product should be refrigerated. Discard unused solution. Protect from light and retain in carton until time of use. Store upright.
Store under refrigeration between 2° to 8°C (36° to 46°F).
REFERENCES
- OSHA Hazardous Drugs, OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
Rev. D 3/2013
Manufactured by:
Teva Pharmaceuticals USA
Sellersville, PA 18960
PRINCIPAL DISPLAY PANEL
Vincasar® PFS 1 mg/mL Single Dose Vial Carton Text
NDC 0703-4402-11 Rx only
Vincasar PFS®
(vinCRIStine sulfate
injection, USP)
PRESERVATIVE FREE SOLUTION
1 mg/mL
FOR INTRAVENOUS USE ONLY
FATAL IF GIVEN BY OTHER ROUTES
1
Single Dose Vial
REFRIGERATE
Protect From Light
TEVA
PRINCIPAL DISPLAY PANEL

Vincasar PFS® 2 mg/2 mL Vial Carton Text
NDC 0703-4412-11 Rx only
Vincasar PFS®
(vinCRIStine sulfate
injection, USP)
PRESERVATIVE FREE SOLUTION
2 mg/2 mL
FOR INTRAVENOUS USE ONLY
FATAL IF GIVEN BY OTHER ROUTES
2
Single Dose Vial
REFRIGERATE
Protect From Light
TEVA
Vincasar PFSvinCRIStine sulfate INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vincasar PFSvinCRIStine sulfate INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||