Venlafaxine Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- VENLAFAXINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- VENLAFAXINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- VENLAFAXINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsAntidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of venlafaxine hydrochloride extended-release capsules or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Venlafaxine hydrochloride extended-release capsules are not approved for use in pediatric patients (see WARNINGS, Clinical Worsening and Suicide Risk; PRECAUTIONS, Information for Patients; and PRECAUTIONS, Pediatric Use).
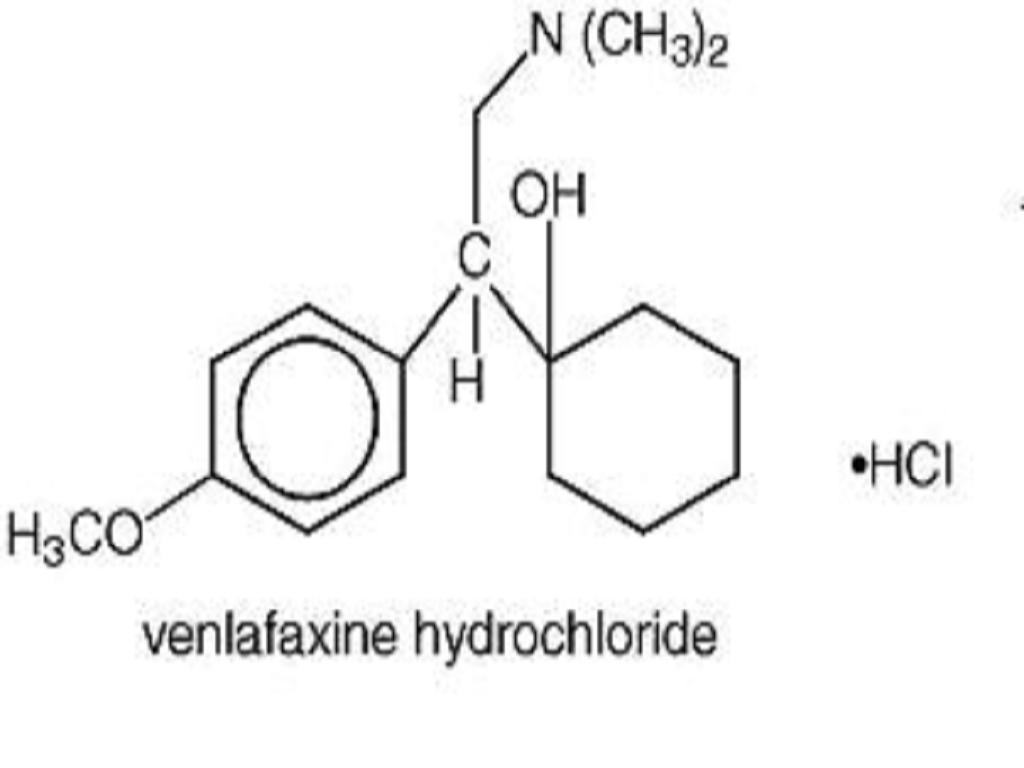
VENLAFAXINE HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
Absorption
Metabolism and Excretion
Special Populations
Age and gender
Extensive/poor metabolizers
Liver disease
Renal disease
Clinical Trials
Major Depressive Disorder
INDICATIONS & USAGE
Major Depressive DisorderVENLAFAXINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Clinical Worsening and Suicide RiskScreening Patients for Bipolar Disorder
Potential for Interaction With Monoamine Oxidase Inhibitors
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-Like Reactions
Sustained Hypertension
Table 2: Number (%) of Sustained Elevations in SDBP in Venlafaxine Hydrochloride Extended-Release Capsules Premarketing Studies by Indication
Elevations in Systolic and Diastolic Blood Pressure
**
Mydriasis
PRECAUTIONS
GeneralDiscontinuation of Treatment With Venlafaxine Hydrochloride Extended-Release Capsules
Insomnia and Nervousness
Changes in Weight
Adult patients
Pediatric patients
The risks associated with longer-term venlafaxine hydrochloride extended-release capsule use were assessed in an open-label MDD study of children and adolescents who received venlafaxine hydrochloride extended-release capsules for up to six months. The children and adolescents in the study had increases in weight that were less than expected based on data from age- and sex-matched peers. The difference between observed weight gain and expected weight gain was larger for children (< 12 years old) than for adolescents (12 years old).
Changes in Height
Pediatric patients
During the eight-week placebo-controlled MDD studies, venlafaxine hydrochloride extended-release capsule-treated patients grew an average of 0.8 cm (n = 146), while placebo-treated patients grew an average of 0.7 cm (n = 147). In the six-month, open-label MDD study, children and adolescents had height increases that were less than expected based on data from age- and sex-matched peers. The difference between observed growth rates and expected growth rates was larger for children (< 12 years old) than for adolescents (12 years old).
Changes in Appetite
Adult patients
Treatment-emergent anorexia was more commonly reported for venlafaxine hydrochloride extended-release capsule-treated (8%) than placebo-treated patients (4%) in the pool of short-term, double-blind, placebo-controlled major depressive disorder studies. The discontinuation rate for anorexia associated with venlafaxine hydrochloride extended-release capsules was 1.0% in major depressive disorder studies.
Pediatric patients
Decreased appetite has been observed in pediatric patients receiving venlafaxine hydrochloride extended-release capsules. In the placebo-controlled trials for MDD, 10% of patients aged 6 to 17 treated with venlafaxine hydrochloride extended-release capsules for up to eight weeks and 3% of patients treated with placebo reported treatment-emergent anorexia (decreased appetite). None of the patients receiving venlafaxine hydrochloride extended-release capsules discontinued for anorexia or weight loss.
Activation of Mania/Hypomania
During premarketing major depressive disorder studies, mania or hypomania occurred in 0.3% of venlafaxine hydrochloride extended-release capsule-treated patients and no placebo patients. In all premarketing major depressive disorder trials with venlafaxine hydrochloride tablets (immediate release), mania or hypomania occurred in 0.5% of venlafaxine-treated patients compared with no placebo patients. Mania/hypomania has also been reported in a small proportion of patients with mood disorders who were treated with other marketed drugs to treat major depressive disorder. As with all drugs effective in the treatment of major depressive disorder, venlafaxine hydrochloride extended-release capsules should be used cautiously in patients with a history of mania.
Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs and SNRIs, including venlafaxine hydrochloride extended-release capsules. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients taking diuretics or who are otherwise volume depleted may be at greater risk (see PRECAUTIONS, Geriatric Use). Discontinuation of venlafaxine hydrochloride extended-release capsules should be considered in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
Seizures
During premarketing experience, no seizures occurred among 705 venlafaxine hydrochloride extended-release capsule-treated patients in the major depressive disorder studies. In all premarketing major depressive disorder trials with venlafaxine hydrochloride tablets (immediate release), seizures were reported at various doses in 0.3% (8/3082) of venlafaxine-treated patients. Venlafaxine hydrochloride extended-release capsules, like many antidepressants, should be used cautiously in patients with a history of seizures and should be discontinued in any patient who develops seizures.
Abnormal Bleeding
SSRIs and SNRIs, including venlafaxine hydrochloride extended-release capsules, may increase the risk of bleeding events. Concomitant use of aspirin, non-steroidal anti-inflammatory drugs, warfarin, and other anti-coagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs and SNRIs use have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages.
Patients should be cautioned about the risk of bleeding associated with the concomitant use of venlafaxine hydrochloride extended-release capsules and NSAIDs, aspirin, or other drugs that affect coagulation.
Serum Cholesterol Elevation
Clinically relevant increases in serum cholesterol were recorded in 5.3% of venlafaxine-treated patients and 0.0% of placebo-treated patients treated for at least 3 months in placebo-controlled trials (see ADVERSE REACTIONS, Laboratory Changes). Measurement of serum cholesterol levels should be considered during long-term treatment.
Interstitial Lung Disease and Eosinophilic Pneumonia
Interstitial lung disease and eosinophilic pneumonia associated with venlafaxine therapy have been rarely reported. The possibility of these adverse events should be considered in venlafaxine-treated patients who present with progressive dyspnea, cough or chest discomfort. Such patients should undergo a prompt medical evaluation, and discontinuation of venlafaxine therapy should be considered.
Use in Patients With Concomitant Illness
Premarketing experience with venlafaxine in patients with concomitant systemic illness is limited. Caution is advised in administering venlafaxine hydrochloride extended-release capsules to patients with diseases or conditions that could affect hemodynamic responses or metabolism.
Venlafaxine has not been evaluated or used to any appreciable extent in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were systematically excluded from many clinical studies during venlafaxine's premarketing testing. The electrocardiograms were analyzed for 275 patients who received venlafaxine hydrochloride extended-release capsules and 220 patients who received placebo in 8 to 12 week double-blind, placebo-controlled trials in major depressive disorder. The mean change from baseline in corrected QT interval (QTc) for venlafaxine hydrochloride extended-release capsule-treated patients in major depressive disorder studies was increased relative to that for placebo-treated patients (increase of 4.7 msec for venlafaxine hydrochloride extended-release capsules and decrease of 1.9 msec for placebo).
In these same trials, the mean change from baseline in heart rate for venlafaxine hydrochloride extended-release capsule-treated patients in the major depressive disorder studies was significantly higher than that for placebo (a mean increase of 4 beats per minute for venlafaxine hydrochloride extended-release capsules and 1 beat per minute for placebo).
In a flexible-dose study, with venlafaxine hydrochloride tablet (immediate release) doses in the range of 200 to 375 mg/day and mean dose greater than 300 mg/day, venlafaxine hydrochloride tablet-treated patients had a mean increase in heart rate of 8.5 beats per minute compared with 1.7 beats per minute in the placebo group.
As increases in heart rate were observed, caution should be exercised in patients whose underlying medical conditions might be compromised by increases in heart rate (e.g., patients with hyperthyroidism, heart failure, or recent myocardial infarction).
Evaluation of the electrocardiograms for 769 patients who received venlafaxine hydrochloride tablets (immediate release) in 4 to 6 week double-blind, placebo-controlled trials showed that the incidence of trial-emergent conduction abnormalities did not differ from that with placebo.
In patients with renal impairment (GFR = 10 to 70 mL/min) or cirrhosis of the liver, the clearances of venlafaxine and its active metabolites were decreased, thus prolonging the elimination half-lives of these substances. A lower dose may be necessary (see DOSAGE AND ADMINISTRATION). Venlafaxine hydrochloride extended-release capsules, like all drugs effective in the treatment of major depressive disorder, should be used with caution in such patients.
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
Interference With Cognitive and Motor Performance
Concomitant Medication
Alcohol
Allergic Reactions
Pregnancy
Nursing
Mydriasis
LABORATORY TESTS
DRUG INTERACTIONS
Alcohol
Cimetidine
Diazepam
Haloperidol
Lithium
Drugs Highly Bound to Plasma Proteins
Drugs That Interfere With Hemostasis (e.g., NSAIDs, Aspirin, and Warfarin)
Drugs That Inhibit Cytochrome P450 Isoenzymes
CYP2D6 inhibitors
Ketoconazole
Venlafaxine AUC increased by 21% in EM subjects and 70% in PM subjects (range in PMs - 2% to 206%), and AUC values for ODV increased by 23% and 33% in EM and PM (range in PMs - 38% to 105%) subjects, respectively. Combined AUCs of venlafaxine and ODV increased on average by approximately 23% in EMs and 53% in PMs (range in PMs - 4% to 134%).
Concomitant use of CYP3A4 inhibitors and venlafaxine may increase levels of venlafaxine and ODV. Therefore, caution is advised if a patient's therapy includes a CYP3A4 inhibitor and venlafaxine concomitantly.
Drugs Metabolized by Cytochrome P450 Isoenzymes
CYP2D6
In vitro studies indicate that venlafaxine is a relatively weak inhibitor of CYP2D6. These findings have been confirmed in a clinical drug interaction study comparing the effect of venlafaxine with that of fluoxetine on the CYP2D6-mediated metabolism of dextromethorphan to dextrorphan.
Imipramine - Venlafaxine did not affect the pharmacokinetics of imipramine and 2-OH-imipramine. However, desipramine AUC, Cmax, and Cmin increased by about 35% in the presence of venlafaxine. The 2-OH-desipramine AUC's increased by at least 2.5 fold (with venlafaxine 37.5 mg q12h) and by 4.5 fold (with venlafaxine 75 mg q12h). Imipramine did not affect the pharmacokinetics of venlafaxine and ODV. The clinical significance of elevated 2-OH-desipramine levels is unknown.
Metoprolol - Concomitant administration of venlafaxine (50 mg every 8 hours for 5 days) and metoprolol (100 mg every 24 hours for 5 days) to 18 healthy male subjects in a pharmacokinetic interaction study for both drugs resulted in an increase of plasma concentrations of metoprolol by approximately 30 to 40% without altering the plasma concentrations of its active metabolite,Metoprolol did not alter the pharmacokinetic profile of venlafaxine or its active metabolite, O-desmethylvenlafaxine.
Venlafaxine appeared to reduce the blood pressure lowering effect of metoprolol in this study. The clinical relevance of this finding for hypertensive patients is unknown. Caution should be exercised with coadministration of venlafaxine and metoprolol.
Venlafaxine treatment has been associated with dose-related increases in blood pressure in some patients. It is recommended that patients receiving venlafaxine hydrochloride extended-release capsules have regular monitoring of blood pressure (see WARNINGS).
Risperidone - Venlafaxine administered under steady-state conditions at 150 mg/day slightly inhibited the CYP2D6-mediated metabolism of risperidone (administered as a single 1 mg oral dose) to its active metabolite, 9-hydroxyrisperidone, resulting in an approximate 32% increase in risperidone AUC. However, venlafaxine coadministration did not significantly alter the pharmacokinetic profile of the total active moiety (risperidone plus 9-hydroxyrisperidone).
CYP3A4
Venlafaxine did not inhibit CYP3A4 in vitro. This finding was confirmed in vivo by clinical drug interaction studies in which venlafaxine did not inhibit the metabolism of several CYP3A4 substrates, including alprazolam, diazepam, and terfenadine.
Indinavir - In a study of 9 healthy volunteers, venlafaxine administered under steady-state conditions at 150 mg/day resulted in a 28% decrease in the AUC of a single 800 mg oral dose of indinavir and a 36% decrease in indinavir Cmax. Indinavir did not affect the pharmacokinetics of venlafaxine and ODV. The clinical significance of this finding is unknown.
CYP1A2
Venlafaxine did not inhibit CYP1A2 in vitro. This finding was confirmed in vivo by a clinical drug interaction study in which venlafaxine did not inhibit the metabolism of caffeine, a CYP1A2 substrate.
CYP2C9
Venlafaxine did not inhibit CYP2C9 in vitro. In vivo, venlafaxine 75 mg by mouth every 12 hours did not alter the pharmacokinetics of a single 500 mg dose of tolbutamide or the CYP2C9 mediated formation of 4-hydroxy-tolbutamide.
CYP2C19
Venlafaxine did not inhibit the metabolism of diazepam, which is partially metabolized by CYP2C19 (see Diazepam above).
Monoamine Oxidase Inhibitors
See CONTRAINDICATIONS and WARNINGS.
CNS-Active Drugs
The risk of using venlafaxine in combination with other CNS-active drugs has not been systematically evaluated (except in the case of those CNS-active drugs noted above). Consequently, caution is advised if the concomitant administration of venlafaxine and such drugs is required.
Serotonergic Drugs
Based on the mechanism of action of venlafaxine hydrochloride extended-release capsules and the potential for serotonin syndrome, caution is advised when venlafaxine hydrochloride extended-release capsules are coadministered with other drugs that may affect the serotonergic neurotransmitter systems, such as triptans, SSRIs, other SNRIs, linezolid (an antibiotic which is a reversible non-selective MAOI), lithium, tramadol, or St. John's Wort (see WARNINGS, Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-Like Reactions). If concomitant treatment of venlafaxine hydrochloride extended-release capsules with these drugs is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases (see WARNINGS, Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-Like Reactions). The concomitant use of venlafaxine hydrochloride extended-release capsules with tryptophan supplements is not recommended (see WARNINGS, Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-Like Reactions).
Triptans
There have been rare postmarketing reports of serotonin syndrome with use of an SSRI and a triptan. If concomitant treatment of venlafaxine hydrochloride extended-release capsules with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases (see WARNINGS, Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-Like Reactions).
Electroconvulsive Therapy
There are no clinical data establishing the benefit of electroconvulsive therapy combined with venlafaxine hydrochloride extended-release capsule treatment.
Postmarketing Spontaneous Drug Interaction Reports
See ADVERSE REACTIONS, Postmarketing Reports.
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
CarcinogenesisMutagenesis
Impairment of Fertility
PREGNANCY
Teratogenic Effects
Pregnancy category C
Nonteratogenic Effects
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
VENLAFAXINE HYDROCHLORIDE ADVERSE REACTIONS
Adverse Findings Observed in Short-Term, Placebo-Controlled Studies With Venlafaxine Hydrochloride Extended-Release Capsules
Adverse Events Associated With Discontinuation of Treatment
*
*Percentage of Patients Discontinuing Due to Adverse EventAdverse EventMajor Depressive Disorder IndicationVenlafaxine Hydrochloride Extended-Release CapsulesPlacebon = 357n = 285Digestive SystemNausea4%< 1%Anorexia1%< 1%Dry Mouth1%0%Nervous SystemDizziness2%1%Insomnia1%< 1%Somnolence2%< 1%
Adverse Events Occurring at an Incidence of 2% or More Among Venlafaxine Hydrochloride Extended-Release Capsule-Treated Patients
The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side effect incidence rate in the population studied.
Commonly Observed Adverse Events From Table 7
Major depressive disorder
Note in particular the following adverse events that occurred in at least 5% of the venlafaxine hydrochloride extended-release capsule patients and at a rate at least twice that of the placebo group for all placebo-controlled trials for the major depressive disorder indication (Table 7): Abnormal ejaculation, gastrointestinal complaints (nausea, dry mouth, and anorexia), CNS complaints (dizziness, somnolence, and abnormal dreams), and sweating. In the two U.S. placebo-controlled trials, the following additional events occurred in at least 5% of venlafaxine hydrochloride extended-release capsule-treated patients (n = 192) and at a rate at least twice that of the placebo group: Abnormalities of sexual function (impotence in men, anorgasmia in women, and libido decreased), gastrointestinal complaints (constipation and flatulence), CNS complaints (insomnia, nervousness, and tremor), problems of special senses (abnormal vision), cardiovascular effects (hypertension and vasodilatation), and yawning.
Table 7: Treatment-Emergent Adverse Event Incidence in Short-Term Placebo-Controlled Venlafaxine Hydrochloride Extended-Release Capsule Clinical Trials in Patients With Major Depressive Disorder*,
*Incidence, rounded to the nearest %, for events reported by at least 2% of patients treated with venlafaxine hydrochloride extended-release capsules, except the following events which had an incidence equal to or less than placebo: abdominal pain, accidental injury, anxiety, back pain, bronchitis, diarrhea, dysmenorrhea, dyspepsia, flu syndrome, headache, infection, pain, palpitation, rhinitis, and sinusitis.< 1% indicates an incidence greater than zero but less than 1%.Mostlyhot flashes.Mostlyvivid dreams,nightmares,andincreased dreaming.Mostlyblurred visionanddifficulty focusing eyes.#Mostlydelayed ejaculation.Incidence is based on the number of male patients.Mostlydelayed orgasmoranorgasmia.Incidence is based on the number of female patients.Body System% Reporting EventVenlafaxine Hydrochloride Extended-Release CapsulesPlaceboPreferred Term(n = 357)(n = 285)Body as a WholeAsthenia8%7%Cardiovascular SystemVasodilatation4%2%Hypertension4%1%Digestive SystemNausea31%12%Constipation8%5%Anorexia8%4%Vomiting4%2%Flatulence4%3%Metabolic/NutritionalWeight Loss3%0%Nervous SystemDizziness20%9%Somnolence17%8%Insomnia17%11%Dry Mouth12%6%Nervousness10%5%Abnormal Dreams7%2%Tremor5%2%Depression3%< 1%Paresthesia3%1%Libido Decreased3%< 1%Agitation3%1%Respiratory SystemPharyngitis7%6%Yawn3%0%SkinSweating14%3%Special SensesAbnormal Vision4%< 1%Urogenital SystemAbnormal Ejaculation (male)#,16%< 1%Impotence4%< 1%Anorgasmia (female),3%< 1%
Vital Sign Changes
Venlafaxine hydrochloride extended-release capsule treatment for up to 12 weeks in premarketing placebo-controlled major depressive disorder trials was associated with a mean final on-therapy increase in pulse rate of approximately 2 beats per minute, compared with 1 beat per minute for placebo (see WARNINGS, Sustained Hypertension and Elevations in Systolic and Diastolic Blood Pressure for effects on blood pressure).
In a flexible-dose study, with venlafaxine hydrochloride tablet (immediate release) doses in the range of 200 to 375 mg/day and mean dose greater than 300 mg/day, the mean pulse was increased by about 2 beats per minute compared with a decrease of about 1 beat per minute for placebo.
Laboratory Changes
Serum Cholesterol
Venlafaxine hydrochloride extended-release capsule treatment for up to 12 weeks in premarketing placebo-controlled trials for major depressive disorder was associated with a mean final on-therapy increase in serum cholesterol concentration of approximately 1.5 mg/dL compared with a mean final decrease of 7.4 mg/dL for placebo.
Patients treated with venlafaxine hydrochloride tablets (immediate-release) for at least 3 months in placebo-controlled 12 month extension trials had a mean final on-therapy increase in total cholesterol of 9.1 mg/dL compared with a decrease of 7.1 mg/dL among placebo-treated patients. This increase was duration dependent over the study period and tended to be greater with higher doses. Clinically relevant increases in serum cholesterol, defined as 1) a final on-therapy increase in serum cholesterol50 mg/dL from baseline and to a value261 mg/dL, or 2) an average on-therapy increase in serum cholesterol50 mg/dL from baseline and to a value261 mg/dL, were recorded in 5.3% of venlafaxine-treated patients and 0.0% of placebo-treated patients (see PRECAUTIONS, General, Serum Cholesterol Elevation).
ECG Changes
In a flexible-dose study, with venlafaxine hydrochloride tablet (immediate release) doses in the range of 200 to 375 mg/day and mean dose greater than 300 mg/day, the mean change in heart rate was 8.5 beats per minute compared with 1.7 beats per minute for placebo.
(See PRECAUTIONS, Use in Patients With Concomitant Illness.)
Other Adverse Events Observed During the Premarketing Evaluation of Venlafaxine Hydrochloride Tablets and Venlafaxine Hydrochloride Extended-Release Capsules
During their premarketing assessment, multiple doses of venlafaxine hydrochloride extended-release capsules were administered to 705 patients in Phase 3 major depressive disorder studies and venlafaxine hydrochloride tablets were administered to 96 patients. In addition, in premarketing assessment of venlafaxine hydrochloride tablets, multiple doses were administered to 2897 patients in Phase 2 to Phase 3 studies for major depressive disorder. The conditions and duration of exposure to venlafaxine in both development programs varied greatly, and included (in overlapping categories) open and double-blind studies, uncontrolled and controlled studies, inpatient (venlafaxine hydrochloride tablets only) and outpatient studies, fixed-dose, and titration studies. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of untoward events into a smaller number of standardized event categories.
In the tabulations that follow, reported adverse events were classified using a standard COSTART-based Dictionary terminology. The frequencies presented, therefore, represent the proportion of the 7212 patients exposed to multiple doses of either formulation of venlafaxine who experienced an event of the type cited on at least one occasion while receiving venlafaxine. All reported events are included except those already listed in Table 7, and those events for which a drug cause was remote. If the COSTART term for an event was so general as to be uninformative, it was replaced with a more informative term. It is important to emphasize that, although the events reported occurred during treatment with venlafaxine, they were not necessarily caused by it.
Events are further categorized by body system and listed in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring on one or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Body as a whole - Frequent: chest pain substernal, chills, fever, neck pain; Infrequent: face edema, intentional injury, malaise, moniliasis, neck rigidity, pelvic pain, photosensitivity reaction, suicide attempt, withdrawal syndrome; Rare: appendicitis, bacteremia, carcinoma, cellulitis, granuloma.
Cardiovascular system - Frequent: migraine, tachycardia; Infrequent: angina pectoris, arrhythmia, bradycardia, extrasystoles, hypotension, peripheral vascular disorder (mainly cold feet and/or cold hands), postural hypotension, syncope; Rare: aortic aneurysm, arteritis, first-degree atrioventricular block, bigeminy, bundle branch block, capillary fragility, cerebral ischemia, coronary artery disease, congestive heart failure, heart arrest, hematoma, cardiovascular disorder (mitral valve and circulatory disturbance), mucocutaneous hemorrhage, myocardial infarct, pallor, sinus arrhythmia, thrombophlebitis.
Digestive system - Frequent: increased appetite; Infrequent: bruxism, colitis, dysphagia, tongue edema, eructation, esophagitis, gastritis, gastroenteritis, gastrointestinal ulcer, gingivitis, glossitis, rectal hemorrhage, hemorrhoids, melena, oral moniliasis, stomatitis, mouth ulceration; Rare: abdominal distension, biliary pain, cheilitis, cholecystitis, cholelithiasis, esophageal spasms, duodenitis, hematemesis, gastroesophageal reflux disease, gastrointestinal hemorrhage, gum hemorrhage, hepatitis, ileitis, jaundice, intestinal obstruction, liver tenderness, parotitis, periodontitis, proctitis, rectal disorder, salivary gland enlargement, increased salivation, soft stools, tongue discoloration.
Endocrine system - Rare: galactorrhoea, goiter, hyperthyroidism, hypothyroidism, thyroid nodule, thyroiditis.
Hemic and lymphatic system - Frequent: ecchymosis; Infrequent: anemia, leukocytosis, leukopenia, lymphadenopathy, thrombocythemia; Rare: basophilia, bleeding time increased, cyanosis, eosinophilia, lymphocytosis, multiple myeloma, purpura, thrombocytopenia.
Metabolic and nutritional - Frequent: edema, weight gain; Infrequent: alkaline phosphatase increased, dehydration, hypercholesteremia, hyperglycemia, hyperlipidemia, hypokalemia, SGOT (AST) increased, SGPT (ALT) increased, thirst; Rare: alcohol intolerance, bilirubinemia, BUN increased, creatinine increased, diabetes mellitus, glycosuria, gout, healing abnormal, hemochromatosis, hypercalcinuria, hyperkalemia, hyperphosphatemia, hyperuricemia, hypocholesteremia, hypoglycemia, hyponatremia, hypophosphatemia, hypoproteinemia, uremia.
Musculoskeletal system - Infrequent: arthritis, arthrosis, bone spurs, bursitis, leg cramps, myasthenia, tenosynovitis; Rare: bone pain, pathological fracture, muscle cramp, muscle spasms, musculoskeletal stiffness, myopathy, osteoporosis, osteosclerosis, plantar fasciitis, rheumatoid arthritis, tendon rupture.
Nervous system - Frequent: amnesia, confusion, depersonalization, hypesthesia, thinking abnormal, trismus, vertigo; Infrequent: akathisia, apathy, ataxia, circumoral paresthesia, CNS stimulation, emotional lability, euphoria, hallucinations, hostility, hyperesthesia, hyperkinesia, hypotonia, incoordination, libido increased, manic reaction, myoclonus, neuralgia, neuropathy, psychosis, seizure, abnormal speech, stupor, suicidal ideation; Rare: abnormal/changed behavior, adjustment disorder, akinesia, alcohol abuse, aphasia, bradykinesia, buccoglossal syndrome, cerebrovascular accident, feeling drunk, loss of consciousness, delusions, dementia, dystonia, energy increased, facial paralysis, abnormal gait, Guillain-Barre syndrome, homicidal ideation, hyperchlorhydria, hypokinesia, hysteria, impulse control difficulties, motion sickness, neuritis, nystagmus, paranoid reaction, paresis, psychotic depression, reflexes decreased, reflexes increased, torticollis.
Respiratory system - Frequent: cough increased, dyspnea; Infrequent: asthma, chest congestion, epistaxis, hyperventilation, laryngismus, laryngitis, pneumonia, voice alteration; Rare: atelectasis, hemoptysis, hypoventilation, hypoxia, larynx edema, pleurisy, pulmonary embolus, sleep apnea.
Skin and appendages - Frequent: pruritus; Infrequent: acne, alopecia, contact dermatitis, dry skin, eczema, maculopapular rash, psoriasis, urticaria; Rare: brittle nails, erythema nodosum, exfoliative dermatitis, lichenoid dermatitis, hair discoloration, skin discoloration, furunculosis, hirsutism, leukoderma, miliaria, petechial rash, pruritic rash, pustular rash, vesiculobullous rash, seborrhea, skin atrophy, skin hypertrophy, skin striae, sweating decreased.
Special senses - Frequent: abnormality of accommodation, mydriasis, taste perversion; Infrequent: conjunctivitis, diplopia, dry eyes, eye pain, otitis media, parosmia, photophobia, taste loss; Rare: blepharitis, cataract, chromatopsia, conjunctival edema, corneal lesion, deafness, exophthalmos, eye hemorrhage, glaucoma, retinal hemorrhage, subconjunctival hemorrhage, hyperacusis, keratitis, labyrinthitis, miosis, papilledema, decreased pupillary reflex, otitis externa, scleritis, uveitis, visual field defect.
Urogenital system - Frequent: albuminuria, urination impaired; Infrequent: amenorrhea,* cystitis, dysuria, hematuria, kidney calculus, kidney pain, leukorrhea,* menorrhagia,* metrorrhagia,* nocturia, breast pain, polyuria, pyuria, prostatic disorder (prostatitis, enlarged prostate, and prostate irritability),* urinary incontinence, urinary retention, urinary urgency, vaginal hemorrhage,* vaginitis*; Rare: abortion,* anuria, breast discharge, breast engorgement, balanitis,* breast enlargement, endometriosis,* female lactation,* fibrocystic breast, calcium crystalluria, cervicitis,* orchitis,* ovarian cyst,* bladder pain, prolonged erection,* gynecomastia (male),* hypomenorrhea,* kidney function abnormal, mastitis, menopause,* pyelonephritis, oliguria, salpingitis,* urolithiasis, uterine hemorrhage,* uterine spasm,* vaginal dryness.*
* Based on the number of men and women as appropriate.
Postmarketing Reports
Adverse Events
Drug Interactions
There have been reports of elevated clozapine levels that were temporally associated with adverse events, including seizures, following the addition of venlafaxine. There have been reports of increases in prothrombin time, partial thromboplastin time, or INR when venlafaxine was given to patients receiving warfarin therapy.
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceManagement of Overdosage
DOSAGE & ADMINISTRATION
Initial Treatment
Major Depressive Disorder
Switching Patients From Venlafaxine Hydrochloride Tablets
Special Populations
Treatment of Pregnant Women During the Third Trimester
Patients With Hepatic Impairment
Patients With Renal Impairment
Elderly Patients
Maintenance Treatment
Discontinuing Venlafaxine Hydrochloride Extended-Release Capsules
Switching Patients to or From a Monoamine Oxidase Inhibitor
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
MEDICATION GUIDE-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Venlafaxine HydrochlorideVenlafaxine Hydrochloride CAPSULE, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!