Venlafaxine Hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use venlafaxine hydrochloride extended-release tablets safely and effectively. See full prescribing information for venlafaxine hydrochloride extended-release tablets. Venlafaxine Hydrochloride (venlafaxine hydrochloride) Tablets, Extended-Release for Oral useInitial U.S. Approval: 1993 RECENT MAJOR CHANGES5.3BOXED WARNINGSee full prescribing information for complete boxed warning.5.1INDICATIONS AND USAGE Major Depressive Disorder (MDD) (1.1) DOSAGE AND ADMINISTRATION Initial Treatment (2.1) Indication Starting Dose Dose Increase Maximum Dose Major Depressive Disorder 75 mg/day (in some patients, 37.5 mg/day for 4 to 7 days) 75 mg/day increments at intervals of 4 days or longer 225 mg/day Venlafaxine hydrochloride extended-release tablets should be taken as a single daily dose with food in either the morning or evening at the same time each day. (2) Discontinuation: Gradual; individualized as necessary. (2.4) DOSAGE FORMS AND STRENGTHS 37.5 mg, 75mg, and 150 mg tablets (3) CONTRAINDICATIONS Concomitant use of monoamine oxidase inhibitors (4) WARNINGS AND PRECAUTIONS Suicidality: Monitor for clinical worsening and suicide risk. (5.1) Monoamine Oxidase Inhibitors (MAOIs): Serious interactions possible. Concomitant use contraindicated. Avoidance of MAOIs recommended for at least 14 days before starting venlafaxine. A MAOI should not be started within 7 days after stopping venlafaxine. (5.2) Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like reactions: Serotonin syndrome or NMS-like reactions have been reported with SSRIs and SNRIs. Discontinue venlafaxine hydrochloride extended-release tablets and initiate supportive treatment. (5.3) Sustained hypertension may occur. Blood pressure monitoring recommended. (5.4) Mydriasis may occur. Patients with raised intraocular pressure or those at risk of acute narrow-angle glaucoma should be monitored. (5.5) Abrupt discontinuation or dose reduction: Discontinuation symptoms may occur (generally self-limiting; serious symptoms possible). Dose reduction recommended to be gradual. (5.6) Activation of Mania/Hypomania has occurred. (5.11) Symptomatic hyponatremia may occur. (5.12) Seizures have been reported. Use with caution in patients with seizure history. (5.13) Abnormal bleeding (most commonly ecchymosis) has been reported. (5.14) Serum cholesterol: Clinically relevant cholesterol increases may occur. Cholesterol measurements should be considered during long-term therapy. (5.15) Interstitial lung disease and eosinophilic pneumonia have been reported. (5.16) Side Effects To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatchDRUG INTERACTIONS MAOI's: concomitant use contraindicated (4). Avoid MAOI's 14 days before starting venlafaxine and 7 days after stopping venlafaxine (5.2). Cimetidine: Caution in patients with pre-existing hypertension, in elderly patients and patients with hepatic dysfunction. (7.2) Haloperidol: Increase in Haloperidol AUC and Cmax. (7.4) Ketoconazole: Increase in venlafaxine and O-desmethylvenlafaxine AUC and Cmax. Caution when using venlafaxine with substances that inhibit both CYP2D6 and CYP3A4. (7.7) Metoprolol: Possibly reduced blood-pressure lowering effect despite increased metoprolol plasma levels. Caution should be exercised with co-administration of venlafaxine and metoprolol. (7.8) CNS-active drugs: Caution when using venlafaxine with such drugs. (7.10) Serotonergic drugs (e.g., triptans, SSRIs, other SNRIs, linezolid, lithium, tramadol, or St. John's Wort): Potential for serotonin syndrome. Careful patient observation advised. (7.10) Tryptophan supplements: Concomitant use not recommended. (7.10) USE IN SPECIFIC POPULATIONS Pregnancy: Use during pregnancy only if clearly needed. Neonates exposed to venlafaxine in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Benefits and risk of venlafaxine use in the third trimester should be carefully considered. (2.3; 8.1) Nursing: Potential for serious adverse reactions in the infant. Discontinue nursing or drug, considering the importance of the drug to the mother. (8.3) Pediatric use: Not approved for use in pediatric patients. When considering use in a child or adolescent, balance potential risks with clinical need. (8.4) Hepatic impairment: Reduction of total daily dose by 50% recommended in patients with mild to moderate impairment. In patients with cirrhosis, further reduction may be necessary and dosing individualization may be desirable. (2.3; 8.6) Renal impairment: Reduction of daily dose by 25 to 50% recommended. Dosing individualization may be necessary. (2.3; 8.7) Hemodialysis: Reduction of daily dose by 50%. (2.3; 8.7)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: Suicidality and Antidepressants
- 1 VENLAFAXINE HYDROCHLORIDE INDICATIONS AND USAGE
- 2 VENLAFAXINE HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 VENLAFAXINE HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 5.1 Clinical Worsening and Suicide Risk
- 5.2 Potential for Interaction with Monoamine Oxidase Inhibitors
- 5.3 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
- 5.4 Sustained Hypertension
- 5.5 Mydriasis
- 5.6 Discontinuation of Treatment with Venlafaxine Hydrochloride Extended-Release Tablets
- 5.7 Insomnia and Nervousness
- 5.8 Changes in Weight
- 5.9 Changes in Height
- 5.10 Changes in Appetite
- 5.11 Activation of Mania/Hypomania
- 5.12 Hyponatremia
- 5.13 Seizures
- 5.14 Abnormal Bleeding
- 5.15 Serum Cholesterol Elevation
- 5.16 Interstitial Lung Disease and Eosinophilic Pneumonia
- 5.17 Use in Patients With Heart Disease
- 5.18 Laboratory Tests
- 6 VENLAFAXINE HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 7.1 Alcohol
- 7.2 Cimetidine
- 7.3 Diazepam
- 7.4 Haloperidol
- 7.5 Lithium
- 7.6 Drugs Highly Bound to Plasma Proteins
- 7.7 Drugs that Inhibit Cytochrome P450 Isoenzymes
- 7.8 Drugs Metabolized by Cytochrome P450 Isoenzymes
- 7.9 Monoamine Oxidase Inhibitors
- 7.10 Other CNS-Active Drugs
- 7.11 Drugs that Interfere with Hemostasis (e.g., NSAID's, Aspirin, and Warfarin)
- 7.12 Electroconvulsive Therapy
- 7.13 Postmarketing Spontaneous Drug Interaction Reports
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 VENLAFAXINE HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- 17.9 FDA-Approved Medication Guide
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 37.5 MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 75 MG
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 150 MG
FULL PRESCRIBING INFORMATION
Venlafaxine hydrochloride extended-release tablets(venlafaxine hydrochloride)WARNING: Suicidality and Antidepressants
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of venlafaxine hydrochloride extended-release tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Venlafaxine hydrochloride extended-release tablets are not approved for use in pediatric patients. [See Warnings and Precautions (5.1) and Patient Counseling Information (17.1) ]
1 INDICATIONS AND USAGE
1.1 Major Depressive Disorder
see Clinical Studies (14.1)
2 DOSAGE AND ADMINISTRATION
2.1 Initial Treatment
see Clinical Studies (14)
See Warnings and Precautions (5.18)
2.2 Maintenance Treatment
see Clinical Studies (14)
2.3 Special Populations
see Use in Specific Populations (8.1)
see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)
see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)
2.4 Discontinuing Venlafaxine Hydrochloride Extended-Release Tablets
Symptoms associated with discontinuation of venlafaxine hydrochloride extended-release capsules, other SNRI's, and SSRI's have been reported [see Warnings and Precautions (5.6)]. Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate. In clinical trials with venlafaxine hydrochloride extended-release capsules, tapering was achieved by reducing the daily dose by 75 mg at 1 week intervals. Individualization of tapering may be necessary.
2.5 Switching Patients from Venlafaxine Hydrochloride Immediate-Release Tablets
Depressed patients who are currently being treated at a therapeutic dose with venlafaxine hydrochloride immediate-release tablets may be switched to venlafaxine hydrochloride extended-release tablets at the nearest equivalent dose (mg/day), e.g., 37.5 mg venlafaxine two-times-a-day to 75 mg venlafaxine hydrochloride extended-release tablets once daily. However, individual dosage adjustments may be necessary.
2.6 Switching Patients To or From a Monoamine Oxidase Inhibitor
see Contraindications (4) and Warnings and Precautions (5.2)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
Concomitant use in patients taking monoamine oxidase inhibitors (MAOIs) is contraindicated [see Warnings and Precautions (5.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Clinical Worsening and Suicide Risk
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated |
|---|---|
| |
Increases Compared to Placebo |
| <18 |
14 additional cases |
| 18 to 24 |
5 additional cases |
| |
Decreases Compared to Placebo |
| 25 to 64 |
1 fewer case |
| ≥65 |
6 fewer cases |
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
see Dosage and Administration (2.5) and Warnings and Precautions (5.7)
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
5.2 Potential for Interaction with Monoamine Oxidase Inhibitors
Adverse reactions, some of which were serious, have been reported in patients who have recently been discontinued from a monoamine oxidase inhibitor (MAOI) and started on venlafaxine, or who have recently had venlafaxine therapy discontinued prior to initiation of an MAOI. These reactions have included tremor, myoclonus, diaphoresis, nausea, vomiting, flushing, dizziness, hyperthermia with features resembling neuroleptic malignant syndrome, seizures, and death. In patients receiving antidepressants with pharmacological properties similar to venlafaxine in combination with an MAOI, there have also been reports of serious, sometimes fatal, reactions. For a selective serotonin reuptake inhibitor, these reactions have included hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, and mental status changes that include extreme agitation progressing to delirium and coma. Some cases presented with features resembling neuroleptic malignant syndrome. Severe hyperthermia and seizures, sometimes fatal, have been reported in association with the combined use of tricyclic antidepressants and MAOIs. These reactions have also been reported in patients who have recently discontinued these drugs and have been started on an MAOI. The effects of combined use of venlafaxine and MAOIs have not been evaluated in humans or animals. Therefore, because venlafaxine is an inhibitor of both norepinephrine and serotonin reuptake, it is recommended that venlafaxine hydrochloride extended-release tablets not be used in combination with an MAOI, or within at least 14 days of discontinuing treatment with an MAOI. Based on the half-life of venlafaxine, at least 7 days should be allowed after stopping venlafaxine before starting an MAOI.
5.3 Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
see Drug Interactions (7.10)
see Contraindications (4) and Warnings and Precautions (5.2)see Drug Interactions (7.10)
see Drug Interactions (7.10)
5.4 Sustained Hypertension
| Major Depressive Disorder (75 to 375 mg/day) |
|---|
| 19/705 (3) |
| Venlafaxine mg/day | Incidence |
|---|---|
| <100 |
3% |
| >100 to ≤200 |
5% |
| >200 to ≤300 |
7% |
| >300 |
13% |
Elevations in Systolic and Diastolic Blood Pressure
| Venlafaxine Hydrochloride Extended-Release Capsules mg/day | Placebo | |||||
|---|---|---|---|---|---|---|
| ≤75 | >75 | |||||
SSBP |
SDBP |
SSBP | SDBP | SSBP | SDBP | |
| Major Depressive Disorder 8 to 12 weeks |
-0.28 |
0.37 |
2.93 |
3.56 |
-1.08 |
-0.10 |
5.5 Mydriasis
Mydriasis has been reported in association with venlafaxine; therefore patients with raised intraocular pressure or those at risk of acute narrow-angle glaucoma (angle-closure glaucoma) should be monitored [see Patient Counseling Information (17.8)].
5.6 Discontinuation of Treatment with Venlafaxine Hydrochloride Extended-Release Tablets
see Dosage and Administration (2.4)
5.7 Insomnia and Nervousness
| Major Depressive Disorder | ||
|---|---|---|
| Venlafaxine Hydrochloride Extended-Release Capsules | Placebo | |
|
Symptom
|
n = 357
|
n = 285
|
| Insomnia |
17% |
11% |
| Nervousness |
10% |
5% |
5.8 Changes in Weight
see Warnings and Precautions (5.10)
5.9 Changes in Height
Pediatric Patients: During the eight-week placebo-controlled MDD studies, venlafaxine hydrochloride extended-release capsule-treated patients grew an average of 0.8 cm (n = 146), while placebo-treated patients grew an average of 0.7 cm (n = 147). In the six-month, open-label MDD study, children and adolescents had height increases that were less than expected based on data from age- and sex-matched peers. The difference between observed growth rates and expected growth rates was larger for children (<12 years old) than for adolescents (≥12 years old).
5.10 Changes in Appetite
5.11 Activation of Mania/Hypomania
During premarketing major depressive disorder studies, mania or hypomania occurred in 0.3% of patients treated with venlafaxine hydrochloride extended-release capsules and 0% placebo patients. In all premarketing major depressive disorder trials with venlafaxine hydrochloride immediate-release tablets, mania or hypomania occurred in 0.5% of venlafaxine-treated patients compared with 0% of placebo patients. Mania/hypomania has also been reported in a small proportion of patients with mood disorders who were treated with other marketed drugs to treat major depressive disorder. As with all drugs effective in the treatment of major depressive disorder, venlafaxine hydrochloride extended-release tablets should be used cautiously in patients with a history of mania.
5.12 Hyponatremia
see Use in Specific Populations (8.5)
5.13 Seizures
During premarketing experience, no seizures occurred among 705 patients treated with venlafaxine hydrochloride extended-release capsules in the major depressive disorder studies. In all premarketing major depressive disorder trials with venlafaxine hydrochloride immediate-release tablets, seizures were reported at various doses in 0.3% (8/3082) of venlafaxine-treated patients. Venlafaxine hydrochloride extended-release tablets, like many antidepressants, should be used cautiously in patients with a history of seizures and should be discontinued in any patient who develops seizures.
5.14 Abnormal Bleeding
5.15 Serum Cholesterol Elevation
Clinically relevant increases in serum cholesterol were recorded in 5.3% of venlafaxine-treated patients and 0% of placebo-treated patients treated for at least 3 months in placebo-controlled trials [see Adverse Reactions (6.1)]. Measurement of serum cholesterol levels should be considered during long-term treatment.
5.16 Interstitial Lung Disease and Eosinophilic Pneumonia
Interstitial lung disease and eosinophilic pneumonia associated with venlafaxine therapy have been rarely reported. The possibility of these adverse reactions should be considered in venlafaxine-treated patients who present with progressive dyspnea, cough or chest discomfort. Such patients should undergo a prompt medical evaluation, and discontinuation of venlafaxine therapy should be considered.
5.17 Use in Patients With Heart Disease
5.18 Laboratory Tests
There are no specific laboratory tests recommended.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
see also Warnings and Precautions (5)
Adverse Reactions Associated with Discontinuation of Treatment
Major Depressive Disorder
Adverse Reactions Occurring at an Incidence of 5% or More
Major Depressive Disorder
Adverse Reactions Occurring at an Incidence of 2% or More Among Patients Treated with Venlafaxine Hydrochloride Extended-Release Capsules
| % Reporting Reaction | ||
|---|---|---|
| Body System Preferred Term |
Venlafaxine Hydrochloride Extended-Release Capsules | Placebo |
|
(n = 357)
|
(n = 285)
|
|
|
Body as a Whole
|
||
| Asthenia |
8% |
7% |
|
Cardiovascular System
|
||
Vasodilatation |
4% |
2% |
| Hypertension |
4% |
1% |
|
Digestive System
|
||
| Nausea |
31% |
12% |
| Constipation |
8% |
5% |
| Anorexia |
8% |
4% |
| Vomiting |
4% |
2% |
| Flatulence |
4% |
3% |
|
Metabolic/Nutritional
|
||
| Weight Loss |
3% |
0% |
|
Nervous System
|
||
| Dizziness |
20% |
9% |
| Somnolence |
17% |
8% |
| Insomnia |
17% |
11% |
| Dry Mouth |
12% |
6% |
| Nervousness |
10% |
5% |
Abnormal Dreams |
7% |
2% |
| Tremor |
5% |
2% |
| Depression |
3% |
<1% |
| Paresthesia |
3% |
1% |
| Libido Decreased |
3% |
<1% |
| Agitation |
3% |
1% |
|
Respiratory System
|
||
| Pharyngitis |
7% |
6% |
| Yawn |
3% |
0% |
|
Skin
|
||
| Sweating |
14% |
3% |
|
Special Senses
|
||
Abnormal Vision |
4% |
<1% |
|
Urogenital System
|
||
Abnormal Ejaculation (male)  |
16% |
<1% |
Impotence |
4% |
<1% |
Anorgasmia (female)  |
3% |
<1% |
Warnings and Precautions (5.4)
Warnings and Precautions (5.17)
Serum Cholesterol
see Warnings and Precautions (5.15)
Serum Triglycerides
See Warnings and Precautions (5.17)
frequentinfrequentrare
Frequent:Infrequent:Rare:
Frequent:Infrequent:Rare:
Frequent:Infrequent:Rare:
Rare:
Frequent:Infrequent:Rare:
Frequent:Infrequent:Rare:
Infrequent:Rare:
Frequent:Infrequent:Rare:
Frequent:Infrequent:Rare:
Frequent:Infrequent:Rare:
Frequent:Infrequent:Rare:
Frequent:Infrequent:Rare:
6.2 Post-Marketing Experience
Voluntary reports of other adverse reactions temporally associated with the use of venlafaxine have been received since market introduction. Because these reactions are reported from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These reports include the following reactions: agranulocytosis, anaphylaxis, aplastic anemia, catatonia, congenital anomalies, impaired coordination and balance, CPK increased, deep vein thrombophlebitis, delirium, EKG abnormalities such as QT prolongation; cardiac arrhythmias including atrial fibrillation, supraventricular tachycardia, ventricular extrasystoles, and rare reports of ventricular fibrillation and ventricular tachycardia, including torsade de pointes; toxic epidermal necrolysis/Stevens-Johnson Syndrome, erythema multiforme, extrapyramidal symptoms (including dyskinesia and tardive dyskinesia), angle-closure glaucoma, hemorrhage (including eye and gastrointestinal bleeding), hepatic reactions (including GGT elevation; abnormalities of unspecified liver function tests; liver damage, necrosis, or failure; and fatty liver), interstitial lung disease, involuntary movements, LDH increased, neutropenia, night sweats, pancreatitis, pancytopenia, panic, prolactin increased, renal failure, rhabdomyolysis, shock-like electrical sensations or tinnitus (in some cases, subsequent to the discontinuation of venlafaxine or tapering of dose), and syndrome of inappropriate antidiuretic hormone secretion (usually in the elderly).
7 DRUG INTERACTIONS
7.1 Alcohol
A single dose of ethanol (0.5 g/kg) had no effect on the pharmacokinetics of venlafaxine or O-desmethylvenlafaxine (ODV) when venlafaxine was administered at 150 mg/day in 15 healthy male subjects. Additionally, administration of venlafaxine in a stable regimen did not exaggerate the psychomotor and psychometric effects induced by ethanol in these same subjects when they were not receiving venlafaxine.
7.2 Cimetidine
Concomitant administration of cimetidine and venlafaxine in a steady-state study for both drugs resulted in inhibition of first-pass metabolism of venlafaxine in 18 healthy subjects. The oral clearance of venlafaxine was reduced by about 43%, and the exposure (AUC) and maximum concentration (Cmax) of the drug were increased by about 60%. However, coadministration of cimetidine had no apparent effect on the pharmacokinetics of ODV, which is present in much greater quantity in the circulation than venlafaxine. The overall pharmacological activity of venlafaxine plus ODV is expected to increase only slightly, and no dosage adjustment should be necessary for most normal adults. However, for patients with pre-existing hypertension, and for elderly patients or patients with hepatic dysfunction, the interaction associated with the concomitant use of venlafaxine and cimetidine is not known and potentially could be more pronounced. Therefore, caution is advised with such patients.
7.3 Diazepam
7.4 Haloperidol
Venlafaxine administered under steady-state conditions at 150 mg/day in 24 healthy subjects decreased total oral-dose clearance (Cl/F) of a single 2 mg dose of haloperidol by 42%, which resulted in a 70% increase in haloperidol AUC. In addition, the haloperidol Cmax increased 88% when coadministered with venlafaxine, but the haloperidol elimination half-life (t1/2) was unchanged. The mechanism explaining this finding is unknown.
7.5 Lithium
The steady-state pharmacokinetics of venlafaxine administered at 150 mg/day were not affected when a single 600 mg oral dose of lithium was administered to 12 healthy male subjects. ODV also was unaffected. Venlafaxine had no effect on the pharmacokinetics of lithium (see also CNS-Active Drugs, below).
7.6 Drugs Highly Bound to Plasma Proteins
Venlafaxine is not highly bound to plasma proteins; therefore, administration of venlafaxine hydrochloride extended-release tablets to a patient taking another drug that is highly protein bound should not cause increased free concentrations of the other drug.
7.7 Drugs that Inhibit Cytochrome P450 Isoenzymes
In vitroin vivosee Clinical Pharmacology (12.3)
maxmax
7.8 Drugs Metabolized by Cytochrome P450 Isoenzymes
CYP2D6
In vitro
maxmin
see Warnings and Precautions (5.5)
in vitroin vivo
max
in vitroin vivo
in vitroIn vivo
Diazepam
7.9 Monoamine Oxidase Inhibitors
See Contraindications (4) and Warnings and Precautions (5.2).
7.10 Other CNS-Active Drugs
see Warnings and Precautions (5.3)see Warnings and Precautions (5.3)see Warnings and Precautions (5.3)
see Warnings and Precautions (5.3)
7.11 Drugs that Interfere with Hemostasis (e.g., NSAID's, Aspirin, and Warfarin)
Serotonin release by platelets plays an important role in hemostasis. Epidemiological studies of the case-control and cohort design that have demonstrated an association between use of psychotropic drugs that interfere with serotonin reuptake and the occurrence of upper gastrointestinal bleeding. These studies have also shown that concurrent use of an NSAID or aspirin may potentiate this risk of bleeding. Altered anticoagulant effects, including increased bleeding, have been reported when SSRI's and SNRI's are coadministered with warfarin. Patients receiving warfarin therapy should be carefully monitored when venlafaxine hydrochloride extended-release tablets are initiated or discontinued. [See Warnings and Precautions (5.14)]
7.12 Electroconvulsive Therapy
There are no clinical data establishing the benefit of electroconvulsive therapy combined with venlafaxine hydrochloride extended-release tablets treatment.
7.13 Postmarketing Spontaneous Drug Interaction Reports
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
2 22
see Warnings and Precautions (5.3)see Dosage and Administration (2)
8.2 Labor and Delivery
The effect of venlafaxine on labor and delivery in humans is unknown.
8.3 Nursing Mothers
Venlafaxine and ODV have been reported to be excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from venlafaxine hydrochloride extended-release tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
see BOXED WARNING and Warnings and Precautions (5.1)
see Warnings and Precautions (5.8, 5.9, and 5.10)
see Warnings and Precautions (5.4 and 5.15)
8.5 Geriatric Use
see Warnings and Precautions (5.12)
see Clinical Pharmacology (12.3)see Dosage and Administration (2.3)
8.6 Patients with Hepatic Impairment
In patients with cirrhosis of the liver, the clearances of venlafaxine and its active metabolite (ODV) were decreased, thus prolonging the elimination half-lives of these substances. A large degree of intersubject variability was noted. [See Clinical Pharmacology (12.3) .] A lower dose and individualization of dosing may be necessary [see Dosage and Administration (2.3)]. Venlafaxine hydrochloride extended-release tablets, like all drugs effective in the treatment of major depressive disorder, should be used with caution in such patients.
8.7 Patients with Renal Impairment
In patients with renal impairment (GFR = 10 to 70 mL/min), the clearances of venlafaxine and its active metabolites were decreased, thus prolonging the elimination half-lives of these substances [see Clinical Pharmacology (12.3)]. It is recommended that the total daily dose be reduced by 25% to 50% in patients with renal impairment. Because there was much individual variability in clearance between patients with renal impairment, individualization of dosage may be desirable in some patients. In patients undergoing hemodialysis, it is recommended that the total daily dose be reduced by 50%. [See Dosage and Administration (2.3) .] Venlafaxine hydrochloride extended-release tablets, like all drugs effective in the treatment of major depressive disorder, should be used with caution in such patients.
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Venlafaxine hydrochloride extended-release tablets (venlafaxine hydrochloride) are not a controlled substance.
9.2 Abuse
While venlafaxine has not been systematically studied in clinical trials for its potential for abuse, there was no indication of drug-seeking behavior in the clinical trials. However, it is not possible to predict on the basis of premarketing experience the extent to which a CNS active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate patients for history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of venlafaxine (e.g., development of tolerance, incrementations of dose, drug-seeking behavior).
9.3 Dependence
In vitro
Dosage and Administration (2.4)Warnings and Precautions (5.6)].
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdosage
Physicians' Desk Reference® PDR
11 DESCRIPTION
17272
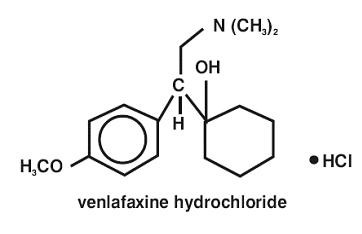
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of the antidepressant action of venlafaxine in humans is believed to be associated with its potentiation of neurotransmitter activity in the CNS. Preclinical studies have shown that venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV), are potent inhibitors of neuronal serotonin and norepinephrine reuptake and weak inhibitors of dopamine reuptake.
12.2 Pharmacodynamics
Venlafaxine and its active metabolite, O-desmethylvenlafaxine (ODV) have no significant affinity for muscarinic cholinergic, H1-histaminergic, or α1-adrenergic receptors in vitro. Pharmacologic activity at these receptors is hypothesized to be associated with the various anticholinergic, sedative, and cardiovascular effects seen with other psychotropic drugs. Venlafaxine and ODV do not possess monoamine oxidase (MAO) inhibitory activity.
12.3 Pharmacokinetics
maxmaxmaxmax
maxmaxmaxmax
maxmax
In vitro
see Dosage and Administration (2)
Dosage and Administration (2.3)Use in Specific Populations (8.6)
see Dosage and Administration (2.3)Use in Specific Populations (8.7)
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
2
in vitroin vivoin vitroin vivo
2
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
760
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F)
17 PATIENT COUNSELING INFORMATION
See 17.9
17.1 Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
17.2 Interference with Cognitive and Motor Performance
Clinical studies were performed to examine the effects of venlafaxine on behavioral performance of healthy individuals. The results revealed no clinically significant impairment of psychomotor, cognitive, or complex behavior performance. However, since any psychoactive drug may impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that venlafaxine therapy does not adversely affect their ability to engage in such activities.
17.3 Concomitant Medication
see Warnings and Precautions (5.3) and Drug Interactions (7.10)
see Warnings and Precautions (5.14) and Drug Interactions (7.11)
17.4 Alcohol
Although venlafaxine has not been shown to increase the impairment of mental and motor skills caused by alcohol, patients should be advised to avoid alcohol while taking venlafaxine.
17.5 Allergic Reactions
Patients should be advised to notify their physician if they develop a rash, hives, or a related allergic phenomenon.
17.6 Pregnancy
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy.
17.7 Nursing
Patients should be advised to notify their physician if they are breast-feeding an infant.
17.8 Mydriasis
Mydriasis (prolonged dilation of the pupils of the eye) has been reported with venlafaxine. Patients should be advised to notify their physician if they have a history of glaucoma or a history of increased intraocular pressure [see Warnings and Precautions (5.5)].
17.9 FDA-Approved Medication Guide
Medication Guide
Antidepressant Medicines, Depression and Other Serious Mental Illnesses, and Suicidal Thoughts or Actions
Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
|
|
- Never stop an antidepressant medicine without first talking to a healthcare provider.
- Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 37.5 MG
NDC 41616-760-81
Venlafaxine Hydrochloride Extended-Release Tablets
37.5 mg
Rx only
90 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH MEDICATION GUIDE PROVIDED SEPARATELY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 75 MG
NDC 41616-759-81
Venlafaxine Hydrochloride Extended-Release Tablets
75 mg
Rx only
90 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH MEDICATION GUIDE PROVIDED SEPARATELY

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - LABEL - 150 MG
NDC 41616-758-81
Venlafaxine Hydrochloride Extended-Release Tablets
150 mg
Rx only
90 TABLETS
SUN PHARMACEUTICAL INDUSTRIES LTD.
PHARMACIST: PLEASE DISPENSE WITH MEDICATION GUIDE PROVIDED SEPARATELY

Venlafaxine HydrochlorideVenlafaxine Hydrochloride TABLET, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Venlafaxine HydrochlorideVenlafaxine Hydrochloride TABLET, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Venlafaxine HydrochlorideVenlafaxine Hydrochloride TABLET, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||