Valacyclovir Hydrochloride
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use valacyclovir hydrochloride safely and effectively. See full prescribing information for valacyclovir tablets, USP. Valacyclovir Tablets, USPInitial U.S. Approval: 1995 RECENT MAJOR CHANGES(5.3)INDICATIONS AND USAGEAdult Patients (1.1) Cold Sores (Herpes Labialis) Genital Herpes Treatment in immunocompetent patients (initial or recurrent episode) Suppression in immunocompetent or HIV-infected patients Reduction of transmission Herpes Zoster Pediatric Patients (1.2) Cold Sores (Herpes Labialis) Chickenpox Limitations of Use (1.3) The efficacy and safety of valacyclovir tablets, USP have not been established in immunocompromised patients other than for the suppression of genital herpes in HIV-infected patients. DOSAGE AND ADMINISTRATION Adult Dosage (2.1) Cold Sores 2 grams every 12 hours for 1 day Genital Herpes Initial episode 1 gram twice daily for 10 days Recurrent episodes 500 mg twice daily for 3 days Suppressive therapy Immunocompetent patients Alternate dose in patients with ≤9 recurrences/yr HIV-infected patients 1 gram once daily 500 mg once daily 500 mg twice daily Reduction of transmission 500 mg once daily Herpes Zoster 1 gram 3 times daily for 7 days Pediatric Dosage (2.2) Cold Sores (≥12 years of age) 2 grams every 12 hours for 1 day Chickenpox (2 to 10% of adult patients treated with valacyclovir hydrochloride and more commonly than in patients treated with placebo are headache, nausea, and abdominal pain. (6.1) The only adverse reaction occurring in >10% of pediatric patients
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 VALACYCLOVIR HYDROCHLORIDE INDICATIONS AND USAGE
- 2 VALACYCLOVIR HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 VALACYCLOVIR HYDROCHLORIDE CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 VALACYCLOVIR HYDROCHLORIDE ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 VALACYCLOVIR HYDROCHLORIDE DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PATIENT INFORMATION
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (100 Tablet Bottle)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg Blister Carton (10 x 10 Unit-dose)
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 gram (100 Tablet Bottle)
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Adult Patients
Cold Sores (Herpes Labialis):
Genital Herpes:Initial Episode:
Recurrent Episodes:
Suppressive Therapy:
Reduction of Transmission: Sexually Transmitted Diseases Treatment Guidelines
Herpes Zoster:
1.2 Pediatric Patients
Cold Sores (Herpes Labialis):
Chickenpox:[see Clinical Studies (14.4)]
1.3 Limitations of Use
- Immunocompromised patients other than for the suppression of genital herpes in HIV-infected patients with a CD4+ cell count ≥100 cells/mm3.
- Patients <12 years of age with cold sores (herpes labialis).
- Patients <2 years of age or ≥18 years of age with chickenpox.
- Patients <18 years of age with genital herpes.
- Patients <18 years of age with herpes zoster.
- Neonates and infants as suppressive therapy following neonatal herpes simplex virus (HSV) infection.
2 DOSAGE AND ADMINISTRATION
- Valacyclovir tablets may be given without regard to meals.
-
Valacyclovir oral suspension (25 mg/mL or 50 mg/mL) may be prepared extemporaneously from 500 mg valacyclovir tablets for use in pediatric patients for whom a solid dosage form is not appropriate [see Dosage and Administration (2.3)].
2.1 Adult Dosing Recommendations
Cold Sores (Herpes Labialis):
Genital Herpes: Initial Episode:
Recurrent Episodes:
Suppressive Therapy:
3
Reduction of Transmission:
Herpes Zoster:
2.2 Pediatric Dosing Recommendations
Cold Sores (Herpes Labialis):
Chickenpox: The recommended dosage of valacyclovir tablets for treatment of chickenpox in immunocompetent pediatric patients 2 to <18 years of age is 20 mg/kg administered 3 times daily for 5 days. The total dose should not exceed 1 gram 3 times daily. Therapy should be initiated at the earliest sign or symptom [see Use in Specific Populations (8.4), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
2.3 Extemporaneous Preparation of Oral Suspension
Ingredients and Preparation per USP-NF:
Prepare Suspension at Time of Dispensing as Follows:
- Prepare SSV according to the USP-NF.
- Using a pestle and mortar, grind the required number of valacyclovir 500 mg tablets until a fine powder is produced (5 valacyclovir tablets for 25 mg/mL suspension; 10 valacyclovir tablets for 50 mg/mL suspension).
- Gradually add approximately 5 mL aliquots of SSV to the mortar and triturate the powder until a paste has been produced. Ensure that the powder has been adequately wetted.
- Continue to add approximately 5 mL aliquots of SSV to the mortar, mixing thoroughly between additions, until a concentrated suspension is produced, to a minimum total quantity of 20 mL SSV and a maximum total quantity of 40 mL SSV for both the 25 mg/mL and 50 mg/mL suspensions.
- Transfer the mixture to a suitable 100 mL measuring flask.
- Transfer the cherry flavor* to the mortar and dissolve in approximately 5 mL of SSV. Once dissolved, add to the measuring flask.
- Rinse the mortar at least 3 times with approximately 5 mL aliquots of SSV, transferring the rinsing to the measuring flask between additions.
- Make the suspension to volume (100 mL) with SSV and shake thoroughly to mix.
- Transfer the suspension to an amber glass medicine bottle with a child-resistant closure.
- The prepared suspension should be labeled with the following information “Shake well before using. Store suspension between 2° to 8°C (36° to 46°F) in a refrigerator. Discard after 28 days.”
2.4 Patients With Renal Impairment
[see Use in Specific Populations (8.5, 8.6), Clinical Pharmacology (12.3)]2
| Indications |
Normal Dosage Regimen (Creatinine Clearance ≥50) |
Creatinine Clearance (mL/min) |
||
| 30-49 |
10-29 |
<10 |
||
|
Cold sores (Herpes labialis)
Do not exceed 1 day of treatment. |
Two 2 gram doses taken 12 hours apart |
Two 1 gram doses taken 12 hours apart |
Two 500 mg doses taken 12 hours apart |
500 mg single dose |
|
Genital herpes: Initial episode |
1 gram every 12 hours |
no reduction |
1 gram every 24 hours |
500 mg every 24 hours |
|
Genital herpes:
Recurrent episode |
500 mg every 12 hours |
no reduction |
500 mg every 24 hours |
500 mg every 24 hours |
|
Genital herpes:
Suppressive therapy Immunocompetent patients Alternate dose for immunocompetent patient with ≤9 recurrences/year HIV-infected patients |
1 gram every 24 hours 500 mg every 24 hours 500 mg every 12 hours |
no reduction no reduction no reduction |
500 mg every 24 hours 500 mg every 48 hours 500 mg every 24 hours |
500 mg every 24 hours 500 mg every 48 hours 500 mg every 24 hours |
| Herpes zoster | 1 gram every 8 hours |
1 gram every 12 hours |
1 gram every 24 hours |
500 mg every 24 hours |
Hemodialysis:
Peritoneal Dialysis:
3 DOSAGE FORMS AND STRENGTHS
- 500 mg: blue, film-coated, capsule shaped tablets with “F 82” on one side and plain on the otherside.
- 1 gram: blue, film-coated, capsule shaped tablets with a partial scorebar on both sides containing “F” on one side and “8” and “3” on the otherside.
4 CONTRAINDICATIONS
[see Adverse Reactions (6.3)]
5 WARNINGS AND PRECAUTIONS
5.1 Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome (TTP/HUS)
5.2 Acute Renal Failure
- Elderly patients with or without reduced renal function. Caution should be exercised when administering valacyclovir hydrochloride to geriatric patients, and dosage reduction is recommended for those with impaired renal function [see Dosage and Administration (2.4), Use in Specific Populations (8.5)].
- Patients with underlying renal disease who received higher than recommended doses of valacyclovir hydrochloride for their level of renal function. Dosage reduction is recommended when administering valacyclovir hydrochloride to patients with renal impairment [see Dosage and Administration (2.4), Use in Specific Populations (8.6)].
- Patients receiving other nephrotoxic drugs. Caution should be exercised when administering valacyclovir hydrochloride to patients receiving potentially nephrotoxic drugs.
- Patients without adequate hydration. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. Adequate hydration should be maintained for all patients.
In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored [see Dosage and Administration (2.4), Adverse Reactions (6.3)].
5.3 Central Nervous System Effects
in[see Adverse Reactions (6.3), Use in Specific Populations (8.5, 8.6)]
6 ADVERSE REACTIONS
- Thrombotic Thrombocytopenic Purpura/Hemolytic Uremic Syndrome [see Warnings and Precautions (5.1)].
- Acute Renal Failure [see Warnings and Precautions (5.2)].
- Central Nervous System Effects [see Warnings and Precautions (5.3)].
6.1 Clinical Trials Experience in Adult Patients
Cold Sores (Herpes Labialis):
Genital Herpes: Initial Episode:
Recurrent Episodes:
Suppressive Therapy: Suppression of Recurrent Genital Herpes in Immunocompetent Adults:
Suppression of Recurrent Genital Herpes in HIV-Infected Patients:
Reduction of Transmission:
Herpes Zoster:
|
a Data were not collected prospectively. LLN = Lower limit of normal. ULN = Upper limit of normal. |
||||||||
| Laboratory Abnormality |
Herpes Zoster |
Genital Herpes Treatment |
Genital Herpes Suppression |
|||||
| Valacyclovir Hydrochloride 1 gram 3 times daily (n = 967) |
Placebo (n = 195) |
Valacyclovir Hydrochloride 1 gram twice daily (n = 1,194) |
Valacyclovir Hydrochloride 500 mg twice daily (n = 1,159) |
Placebo (n = 439) |
Valacyclovir Hydrochloride 1 gram once daily (n = 269) |
Valacyclovir Hydrochloride 500 mg once daily (n = 266) |
Placebo (n =134) |
|
| Hemoglobin (<0.8 x LLN) White blood cells (<0.75 x LLN) Platelet count (<100,000/mm3) AST (SGOT) (>2 x ULN) Serum creatinine (>1.5 x ULN) |
0.8% 1.3% 1% 1% 0.2% |
0% 0.6% 1.2% 0% 0% |
0.3% 0.7% 0.3% 1% 0.7% |
0.2% 0.6% 0.1% a 0% |
0% 0.2% 0.7% 0.5% 0% |
0% 0.7% 0.4% 4.1% 0% |
0.8% 0.8% 1.1% 3.8% 0% |
0.8% 1.5% 1.5% 3% 0% |
6.2 Clinical Trials Experience in Pediatric Patients
Pediatric Patients 12 to <18 Years of Age (Cold Sores):
Pediatric Patients 1 Month to <12 Years of Age:
6.3 Postmarketing Experience
General:
Allergic:[see Contraindications (4)]
CNS Symptoms: [see Warnings and Precautions (5.3), Use in Specific Populations (8.5), (8.6)]
Eye:
Gastrointestinal:
Hepatobiliary Tract and Pancreas:
Renal:[see Warnings and Precautions (5.2), Use in Specific Populations (8.5), (8.6)]
Hematologic:[see Warnings and Precautions (5.1)]
Skin:
7 DRUG INTERACTIONS
[see Clinical Pharmacology (12.3)]
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
in-utero
8.3 Nursing Mothers
max
8.4 Pediatric Use
[see Indications and Usage (1.2), Dosage and Administration (2.2)].
[see Clinical Studies (14.1)]
[see Dosage and Administration (2.2), Adverse Reactions (6.2), Clinical Pharmacology (12.3), Clinical Studies (14.4)]
- <12 years of age with cold sores
- <18 years of age with genital herpes
- <18 years of age with herpes zoster
- <2 years of age with chickenpox
- for suppressive therapy following neonatal HSV infection.
[see Dosage and Administration (2.2), Adverse Reactions (6.2), Clinical Pharmacology (12.3), Clinical Studies (14.4)].
max
max
8.5 Geriatric Use
[see Dosage and Administration (2.4), Warnings and Precautions (5.2, 5.3), Clinical Pharmacology (12.3)]
8.6 Renal Impairment
[see Dosage and Administration (2.4), Warnings and Precautions (5.2, 5.3)]
10 OVERDOSAGE
[see Use in Specific Populations (8.5), (8.6)][see Dosage and Administration (2.4)]
11 DESCRIPTION
L
LH
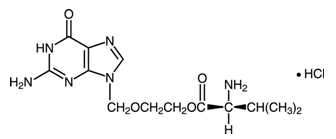
132064a
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
[see Clinical Pharmacology (12.4)]
12.3 Pharmacokinetics
Pharmacokinetics in Adults:Absorption and Bioavailability:L-
max
|
a Administered 4 times daily for 11 days. ND = not done. |
||||
|
Dose |
Single-Dose Administration (N = 8) |
Multiple-Dose Administrationa
(N = 24, 8 per treatment arm) |
||
| Cmax (±SD) (mcg/mL) |
AUC (±SD) (hr•mcg/mL) |
Cmax (±SD) (mcg/mL) |
AUC (±SD) (hr•mcg/mL) |
|
| 100 mg |
0.83 (±0.14) |
2.28 (±0.4) |
ND |
ND |
| 250 mg |
2.15 (±0.5) |
5.76 (±0.6) |
2.11 (±0.33) |
5.66 (±1.09) |
| 500 mg |
3.28 (±0.83) |
11.59 (±1.79) |
3.69 (±0.87) |
9.88 (±2.01) |
| 750 mg |
4.17 (±1.14) |
14.11 (±3.54) |
ND |
ND |
| 1,000 mg |
5.65 (±2.37) |
19.52 (±6.04) |
4.96 (±0.64) |
15.7 (±2.27) |
Distribution:
Metabolism:L
Elimination:
Specific Populations:Renal Impairment:[see Dosage and Administration (2.4), Use in Specific Populations (8.5), (8.6)]
22
Hepatic Impairment:
HIV Disease: 3
Geriatrics: [see Dosage and Administration (2.4), Use in Specific Populations (8.5), (8.6)]
Pediatrics: [see Adverse Reactions (6.2), Use in Specific Populations (8.4)]
|
a Historical estimates using pediatric pharmacokinetic sampling schedule. |
||||
| Parameter |
Pediatric Patients (20 mg/kg Oral Suspension) |
Adults 1 gram Solid Dose of Valacyclovir Hydrochloridea (N = 15) |
||
| 1 - <2 yr (N = 6) |
2 - <6 yr (N = 12) |
6 - <12 yr (N = 8) |
||
| AUC (mcg•hr/mL) Cmax (mcg/mL) |
14.4 (±6.26) 4.03 (±1.37) |
10.1 (±3.35) 3.75 (±1.14) |
13.1 (±3.43) 4.71 (±1.2) |
17.2 (±3.1) 4.72 (±1.37) |
Drug Interactions:
Antacids: 3+++
Cimetidine: max
Cimetidine Plus Probenecid: max
Digoxin:
Probenecid: max
Thiazide Diuretics:
12.4 Microbiology
Mechanism of Action:in vivo
Antiviral Activities:50505050
Resistance:
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
[see Clinical Pharmacology (12.3)]
in vitro
14 CLINICAL STUDIES
14.1 Cold Sores (Herpes Labialis)
14.2 Genital Herpes Infections
Initial Episode:
Recurrent Episodes:
Suppressive Therapy:
|
a Includes lost to follow-up, discontinuations due to adverse events, and consent withdrawn. |
||||||
| Outcome |
6 Months |
12 Months |
||||
| Valacyclovir Hydrochloride 1 gram once daily (n = 269) |
Oral acyclovir 400 mg twice daily (n = 267) |
Placebo (n = 134) |
Valacyclovir Hydrochloride 1 gram once daily (n = 269) |
Oral acyclovir 400 mg twice daily (n = 267) |
Placebo (n = 134) |
|
| Recurrence free |
55% |
54% |
7% |
34% |
34% |
4% |
| Recurrences |
35% |
36% |
83% |
46% |
46% |
85% |
| Unknowna
|
10% |
10% |
10% |
19% |
19% |
10% |
1033333
|
a Includes lost to follow-up, discontinuations due to adverse events, and consent withdrawn. |
||
| Outcome |
Valacyclovir Hydrochloride 500 mg twice daily (n = 194) |
Placebo (n = 99) |
| Recurrence free |
65% |
26% |
| Recurrences |
17% |
57% |
| Unknowna
|
18% |
17% |
Reduction of Transmission of Genital Herpes:
|
a Results show reductions in risk of 75% (symptomatic HSV-2 acquisition), 50% (HSV-2 seroconversion), and 48% (overall HSV-2 acquisition) with valacyclovir hydrochloride versus placebo. Individual results may vary based on consistency of safer sex practices. |
||
| Endpoint |
Valacyclovir Hydrochloridea
(n = 743) |
Placebo (n = 741) |
| Symptomatic HSV-2 acquisition |
4 (0.5%) |
16 (2.2%) |
| HSV-2 seroconversion |
12 (1.6%) |
24 (3.2%) |
| Overall HSV-2 acquisition |
14 (1.9%) |
27 (3.6%) |
14.3 Herpes Zoster
14.4 Chickenpox
max[see Use in Specific Populations (8.4)]
16 HOW SUPPLIED/STORAGE AND HANDLING
Valacyclovir Tablets USP, 500 mg
Valacyclovir Tablets USP, 1 gram
Store at oooo
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling.
17.1 Importance of Adequate Hydration
Patients should be advised to maintain adequate hydration.
17.2 Cold Sores (Herpes Labialis)
17.3 Genital Herpes
17.4 Herpes Zoster
17.5 Chickenpox
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PATIENT INFORMATION
Valacyclovir Tablets, USP
What are valacyclovir tablets?
- to treat cold sores (also called fever blisters or herpes labialis)
- to treat shingles (also called herpes zoster)
- to treat or control genital herpes outbreaks in adults with normal immune systems
- to control genital herpes outbreaks in adults infected with the human immunodeficiency virus (HIV) with CD4+ cell count greater than 100 cells/mm3
- with safer sex practices to lower the chances of spreading genital herpes to others. Even with safer sex practices, it is still possible to spread genital herpes.
- Do not have sexual contact with your partner when you have any symptom or outbreak of genital herpes.
- Use a condom made of latex or polyurethane whenever you have sexual contact.
- to treat cold sores (for children ≥12 years of age)
- to treat chickenpox (for children 2 to <18 years of age).
Valacyclovir tablets do not cure herpes infections
What are cold sores, chickenpox, shingles, and genital herpes?
Cold sores
Chickenpox
Shingles
Genital herpessafer sex practices
- Do not have sexual contact with your partner when you have any symptom or outbreak of genital herpes.
- Use a condom made of latex or polyurethane whenever you have sexual contact.
Who should not take valacyclovir tablets?
Do not take valacyclovir tablets
Before taking valacyclovir tablets, tell your healthcare provider:
About all your medical conditions
- if you have had a bone marrow transplant or kidney transplant, or if you have advanced HIV disease or “AIDS”. Patients with these conditions may have a higher chance for getting a blood disorder called thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS). TTP/HUS can result in death.
- if you have kidney problems. Patients with kidney problems may have a higher chance for getting side effects or more kidney problems with valacyclovir tablets. Your healthcare provider may give you a lower dose of valacyclovir tablets.
- if you are 65 years of age or older. Elderly patients have a higher chance of certain side effects. Also, elderly patients are more likely to have kidney problems. Your healthcare provider may give you a lower dose of valacyclovir tablets.
- if you are pregnant or planning to become pregnant. Talk with your healthcare provider about the risks and benefits of taking prescription drugs (including valacyclovir tablets) during pregnancy.
- if you are breastfeeding. Valacyclovir hydrochloride may pass into your milk and it may harm your baby. Talk with your healthcare provider about the best way to feed your baby if you are taking valacyclovir tablets.
- about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Valacyclovir tablets may affect other medicines, and other medicines may affect valacyclovir tablets. It is a good idea to keep a complete list of all the medicines you take. Show this list to your healthcare provider and pharmacist any time you get a new medicine.
How should I take valacyclovir tablets?
- Do not stop valacyclovir tablets or change your treatment without talking to your healthcare provider.
- Valacyclovir tablets can be taken with or without food.
- If you are taking valacyclovir tablets to treat cold sores, chickenpox, shingles, or genital herpes, you should start treatment as soon as possible after your symptoms start. Valacyclovir tablets may not help you if you start treatment too late.
- If you miss a dose of valacyclovir tablet, take it as soon as you remember and then take your next dose at its regular time. However, if it is almost time for your next dose, do not take the missed dose. Wait and take the next dose at the regular time.
- Do not take more than the prescribed number of valacyclovir tablets each day. Call your healthcare provider right away if you take too much valacyclovir tablets.
What are the possible side effects of valacyclovir tablets?
Kidney failure and nervous system problems are not common, but can be serious in some patients taking valacyclovir tablets. Always tell your healthcare provider if you have kidney problems before taking valacyclovir tablets. Call your doctor right away if you get a nervous system problem while you are taking valacyclovir tablets.
Talk to your healthcare provider if you develop any side effects that concern you.
How should I store valacyclovir tablets?
- Store valacyclovir tablets at room temperature, 20º to 25ºC (68º to 77ºF).
- Store valacyclovir oral suspension between 2o to 8oC (36o to 46oF) in a refrigerator. Discard after 28 days.
- Keep valacyclovir tablets in a tightly closed container.
- Do not keep medicine that is out of date or that you no longer need.
- Keep valacyclovir tablets and all medicines out of the reach of children.
General information about valacyclovir tablets
What are the ingredients in valacyclovir tablets?
Active Ingredient:
Inactive Ingredients:
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg (100 Tablet Bottle)
NDC 65862-448-01
Valacyclovir Tablets, USP
500 mg
PHARMACIST:
Rx only 100 Tablets
AUROBINDO

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 500 mg Blister Carton (10 x 10 Unit-dose)
Rx only NDC 65862-448-10
Valacyclovir Tablets, USP
500 mg
PHARMACIST:
100 (10 blister cards each containing 10 tablets) Tablets
AUROBINDO
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 gram (100 Tablet Bottle)
NDC 65862-449-01
Valacyclovir Tablets, USP
1 gram
PHARMACIST:
Rx only 100 Tablets
AUROBINDO
Valacyclovir HydrochlorideValacyclovir Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Valacyclovir HydrochlorideValacyclovir Hydrochloride TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||