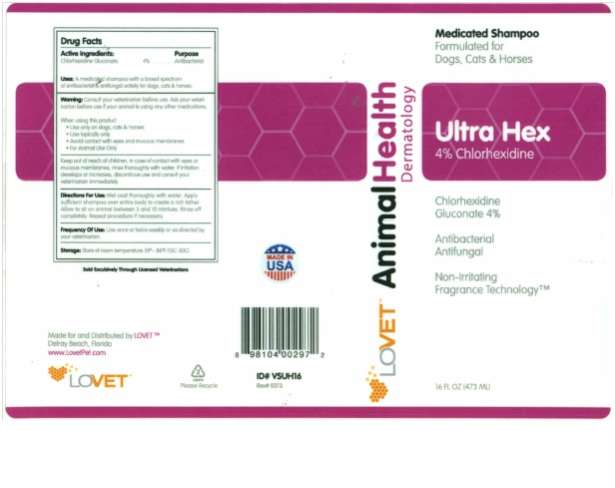
Ultra Hex
Nootie LLC
Custom Veterinary Services, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients : Purpose
Chlorhexidine Gluconate..… 4% ...… Antibacterial
Purpose
Uses: A medicated shampoo with a broad spectrum of antibacterial and antifungal activity for dogs, cats and horses.
Warning: Consult your veterinarian before use. Ask your veterinarian before use if your animal is using any other medications.
When Using this Product
- Use only on dogs, cats and horses
- Use topically only
- Avoid contact with eyes and mucous membranes
- For Animal Use Only
Keep out of reach of children. In case of contact with eyes or mucous membranes, rinse thoroughly with water. If irritation develops or increases, discontinue use and consult your veterinarian immediately.
Directions For Use: Wet coat thoroughly with water. Apply sufficient shampoo over entire body to create a rich lather. Allow to sit on animal between 5 and 10 minutes. Rinse off completely. Repeat procedure if necessary.
Frequency Of Use: Use once or twice weekly or as directed by your veterinarian.
Storage: Store at room temperature 59º– 86ºF (15C-30C)
Sold Exclusively Through Licensed Veterinarians
Made for and Distributed by LOVET
Delray Beach, Florida
www.LovetPet.com
LOVET
ID # VSUH16 Rev# 0313 / VSUH04 Rev#0813
Made In USA
HDPE
Please Recycle
LOVET
Animal Health Dermatology
Medicated Shampoo
Formulated for Dogs, Cats and Horses
Ultra Hex
4% Chlorhexidine
Chlorhexidine Gluconate 4%
Antibacterial
Antifungal
Non-Irritating Fragrance Technology
16 FL oz (473 ML) / 4 FL OZ (118 ML)
Ultra HexChlorhexidine Gluconate SHAMPOO
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||