Tussin DM
Chain Drug Consortium, LLC
AptaPharma Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Keep out of reach of children
- Tussin DM Uses
- Warnings
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Directions
- Tussin DM Other information
- Inactive ingredients
- Questions?
- Product Label
FULL PRESCRIBING INFORMATION
Active ingredients
Drug Facts
Active ingredients (in each 5 mL tsp)
Dextromethorphan HBr, USP 10 mg
Guaifenesin, USP 100 mg
Purpose
Cough Suppressant
Expectorant
Keep out of reach of children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Tussin DM Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- helps loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
Warnings
Do not use
- in a child under 12 years of age
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Stop use and ask a doctor if
cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache.
These could be signs of a serious condition.
If pregnant or breast-feeding
ask a health professional before use.
Directions
- do not take more than 6 doses in any 24-hour period
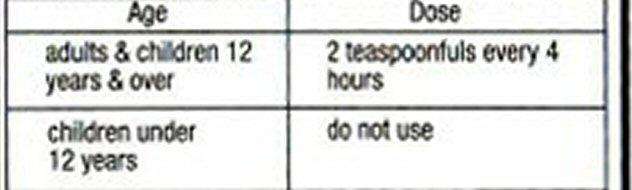
Tussin DM Other information
- store at 20‐25 ° C (68‐77 ° F)
- do not refrigerate
- dosage cup provided
- sodium 3 mg per teaspoonful
- See carton for full labeling
Inactive ingredients
anhydrous citric acid, dextrose, FD and C red no.40, flavor, glycerin, high fructose corn syrup, menthol, purified water, saccharin sodium, sodium benzoate
Questions?
Call weekdays from 9:30 AM to 4:30 PM EST at
1-877-798-5944
Product Label
NDC 68016-855-56
*COMPARE TO THE ACTIVE INGREDIENTS IN ROBITUSSIN® PEAK COLD COUGH and CHEST CONGESTION DM
Premier Value®
Tussin DM
Dextromethorphan HBr
Guaifenesin
COUGH SUPPRESSANT /
EXPECTORANT
NON-DROWSY
Helps to Loosen
Chest Congestion
Cough Formula
for ages 12 and over
8 FL OZ (237 mL)
INDEPENDENTLY TESTED SATISFACTION GUARANTEED
DO NOT USE IF IMPRINTED SHRINK BAND IS MISSING OR BROKEN
*This product is not manufactured or distributed by Pfizer, owner of the registered trademark Robitussin® Peak Cold.
DISTRIBUTED BY:
CHAIN DRUG CONSORTIUM
3301 NW BOCA RATON BLVD
SUITE 101, BOCA RATON, FL 33431
LR-018
LOT: EXP:

Tussin DMDEXTROMETHORPHAN HYDROBROMIDE, GUAIFENESIN LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||