Trihexyphenidyl Hydrochloride
PACK Pharmaceuticals, LLC
Natco Pharma Limited-Pharma Division
Trihexyphenidyl hydrochloride tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- RX Only
- TRIHEXYPHENIDYL HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- TRIHEXYPHENIDYL HYDROCHLORIDE INDICATIONS AND USAGE
- TRIHEXYPHENIDYL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TRIHEXYPHENIDYL HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- TRIHEXYPHENIDYL HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Trihexyphenidyl Hydrochloride Tablets, USP 2 mg-Bottle of 100 tablets
- Trihexyphenidyl Hydrochloride Tablets, USP 2 mg-Bottle of 1000 tablets
- Trihexyphenidyl Hydrochloride Tablets, USP 5 mg-Bottle of 100 tablets
- Trihexyphenidyl Hydrochloride Tablets, USP 5 mg-Bottle of 1000 tablets
FULL PRESCRIBING INFORMATION
RX Only
TRIHEXYPHENIDYL HYDROCHLORIDE DESCRIPTION
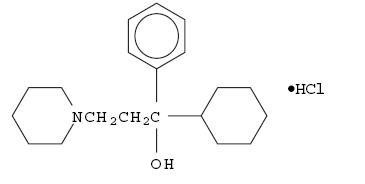
C20H31NO HCl M.W.337.93
Trihexyphenidyl HCl occurs as a white or creamy-white, almost odorless, crystalline powder. It is very slightly soluble in ether and benzene, slightly soluble in water and soluble in methanol.
Trihexyphenidyl Hydrochloride Tablets USP 2 mg and 5 mg contain the following inactive ingredients: magnesium stearate, microcrystalline cellulose and sodium starch glycolate.
CLINICAL PHARMACOLOGY
Trihexyphenidyl HCl exerts a direct inhibitory effect upon the parasympathetic nervous system. It also has a relaxing effect on smooth musculature; exerted both directly upon the muscle tissue itself and indirectly through an inhibitory effect upon the parasympathetic nervous system. Its therapeutic properties are similar to those of atropine although undesirable side effects are ordinarily less frequent and severe than with the latter.
TRIHEXYPHENIDYL HYDROCHLORIDE INDICATIONS AND USAGE
Trihexyphenidyl HCl is indicated as an adjunct in the treatment of all forms of parkinsonism (postencephalitic, arteriosclerotic, and idiopathic). It is often useful as adjuvant therapy when treating these forms of parkinsonism with levodopa. Additionally, it is indicated for the control of extrapyramidal disorders caused by central nervous system drugs such as the dibenzoxazepines, phenothiazines, thioxanthenes, and butyrophenones.
TRIHEXYPHENIDYL HYDROCHLORIDE CONTRAINDICATIONS
Trihexyphenidyl HCl is contraindicated in patients with hypersensitivity to trihexyphenidyl HCl orto any of the tablet ingredients. Trihexyphenidyl HCl is also contraindicated in patients with narrow angle glaucoma. Blindness after long-term use due to narrow angle glaucoma has been reported.
WARNINGS
CONTRAINDICATIONS ADVERSE REACTIONS
Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with dose reduction or discontinuation of trihexyphenidyl. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac dysrhythmias).
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (eg, pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
PRECAUTIONS
General
Since trihexyphenidyl HCl has atropine-like properties, patients on long-term treatment should be carefully monitored for untoward reactions.
( DRUG ABUSE AND DEPENDENCE .)
DOSAGE AND ADMINISTRATION
Information for Patients
Trihexyphenidyl HCl may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or driving a motor vehicle. Patients should be cautioned about operating machinery, including automobiles, until they are reasonably certain that trihexyphenidyl HCl therapy does not adversely affect their ability to engage in such activities.
Because of increased sedative effects, patients should be cautioned to avoid the use of alcohol or other CNS depressants while taking trihexyphenidyl HCl.
( WARNINGS.)
( WARNINGS.)
Drug Interactions
Cannabinoids, barbiturates, opiates, and alcohol may have additive effects with trihexyphenidyl HCl, and thus, an abuse potential exists.
Concurrent use of alcohol or other CNS depressants with trihexyphenidyl HCl may cause increased sedative effects.
Monoamine oxidase inhibitors and tricyclic antidepressants possessing significant anticholinergic activity may intensify the anticholinergic effects of antidyskinetic agents because of the secondary anticholinergic activities of these medications.
Prophylactic administration of anticholinergic agents, such as trihexyphenidyl, as a prevention of drug-induced parkinsonism during neuroleptic therapy is not recommended. There may be an increased risk for the development of tardive dyskinesia during concomitant administration of anticholinergics and neuroleptics (see PRECAUTIONS, General).
The usual dose of either trihexyphenidyl or levodopa may need to be reduced during concomitant therapy, since concomitant administration may increase drug-induced involuntary movements (see DOSAGE AND ADMINISTRATION).
Carcinogenesis, Mutagenesis, Impairment Of Fertility
No carcinogenicity studies or adequate genotoxicity or fertility studies have been conducted for trihexyphenidyl HCl.
Pregnancy
Animal reproduction studies to evaluate teratogenic and embryotoxic potential have not been conducted with trihexyphenidyl HCl. It is also not known whether trihexyphenidyl HCl can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Trihexyphenidyl HCl should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when trihexyphenidyl HCl is administered to a nursing woman.
As with other anticholinergics, trihexyphenidyl may cause suppression of lactation. Therefore, trihexyphenidyl should only be used if the expected benefit to the mother outweighs the potential risk to the infant.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established. (See also ADVERSE REACTIONS.)
Geriatric Use
Sensitivity to the actions of parasympatholytic drugs may increase with age, particularly over the age of 60; therefore, elderly patients generally should be started on low doses of trihexyphenidyl HCl and observed closely. Trihexyphenidyl HCl has been shown to cause some cognitive dysfunctions in the elderly, including confusion and memory impairment. (See ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION .)
TRIHEXYPHENIDYL HYDROCHLORIDE ADVERSE REACTIONS
Minor side effects, such as dryness of the mouth, blurred vision, dizziness, mild nausea or nervousness, will be experienced by 30 to 50 percent of all patients. These sensations, however, are much less troublesome with trihexyphenidyl HCl than with belladonna alkaloids and are usually less disturbing than unalleviated parkinsonism. Such reactions tend to become less pronounced, and even to disappear, as treatment continues. Even before these reactions have remitted spontaneously, they may often be controlled by careful adjustment of dosage form, amount of drug, or interval between doses.
Isolated instances of suppurative parotitis secondary to excessive dryness of the mouth, skin rashes, dilatation of the colon, paralytic ileus, and certain psychiatric manifestations such as delusions, hallucinations, and paranoia, all of which may occur with any of the atropine-like drugs, have been reported rarely with trihexyphenidyl HCl.
Potential side effects associated with the use of any atropine-like drugs, including trihexyphenidyl HCl, include cognitive dysfunctions, including confusion and memory impairment; constipation, drowsiness, urinary hesitancy or retention, tachycardia, dilation of the pupil, increased intraocular pressure, choreiform movements, weakness, vomiting, and headache. Exacerbation of parkinsonism with abrupt treatment withdrawal has been reported. Neuroleptic malignant syndrome with abrupt treatment withdrawal has been reported (see WARNINGS, Neuroleptic Malignant Syndrome).
The occurrence of angle-closure glaucoma in patients receiving trihexyphenidyl HCl has been reported (blindness has been reported in some cases). Paradoxical sinus bradycardia, dry skin, and cycloplegia have been reported.
In addition to adverse events seen in adults, the following adverse events have been reported in the literature in pediatric patients: hyperkinesia, psychosis, forgetfulness, weight loss, restlessness, chorea, and sleep alterations.
DRUG ABUSE AND DEPENDENCE
Although trihexyphenidyl HCl is not classified as a controlled substance, the possibility of abuse should be borne in mind due to its stimulant and euphoriant properties.
OVERDOSAGE
The mean oral LD50 of trihexyphenidyl HCl has been reported to be 365 mg/kg (range, 325 to 410 mg/kg) in mice and 1660 mg/kg (1420 to 1940 mg/kg) in rats. At a dose of 40 mg/kg, dogs have exhibited emesis, restlessness followed by drowsiness, equilibrium disturbances, and mydriasis.
Signs and Symptoms
Overdosage with trihexyphenidyl HCl produces typical central symptoms of atropine intoxication (the central anticholinergic syndrome). Correct diagnosis depends upon recognition of the peripheral signs of parasympathetic blockade, including dilated and sluggish pupils; warm, dry skin; facial flushing; decreased secretions of the mouth, pharynx, nose, and bronchi; foul-smelling breath; elevated temperature; tachycardia, cardiac arrhythmias; decreased bowel sounds; and urinary retention. Neuropsychiatric signs such as delirium, disorientation, anxiety, hallucinations, illusions, confusion, incoherence, agitation, hyperactivity, ataxia, lip smacking and tasting movements, loss of memory, paranoia, combativeness, and seizures may be present. The condition can progress to stupor, coma, paralysis, cardiac and respiratory arrest, and death.
Treatment
Treatment of acute overdose involves symptomatic and supportive therapy. Gastric lavage or other methods to limit absorption should be instituted. A small dose of diazepam or a short- acting barbiturate may be administered if CNS excitation is observed. Phenothiazines are contraindicated because the toxicity may be intensified due to their antimuscarinic action, causing coma. Respiratory support, artificial respiration or vasopressor agents may be necessary. Hyperpyrexia must be reversed, fluid volume replaced and acid-balance maintained. Urinary catheterization may be necessary. It is not known if trihexyphenidyl HCl is dialyzable.
TRIHEXYPHENIDYL HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Dosage should be individualized. The initial dose should be low and then increased gradually, especially in patients over 60 years of age. Whether trihexyphenidyl HCl may best be given before or after meals should be determined by the way the patient reacts. Postencephalitic patients, who are usually more prone to excessive salivation, may prefer to take it after meals and may, in addition, require small amounts of atropine which, under such circumstances, is sometimes an effective adjuvant. If trihexyphenidyl HCl tends to dry the mouth excessively, it may be better to take it before meals, unless it causes nausea. If taken after meals, the thirst sometimes induced can be allayed by mint candies, chewing gum or water.
Abrupt withdrawal of treatment for parkinsonism may result in acute exacerbation of parkinsonism symptoms; therefore, abrupt withdrawal should be avoided.
Abrupt withdrawal of treatment may result in neuroleptic malignant syndrome (NMS) (see WARNINGS ).
Idiopathic Parkinsonism
As initial therapy for parkinsonism, 1 mg of trihexyphenidyl HCl in tablet form may be administered the first day. The dose may then be increased by 2 mg increments at intervals of three to five days, until a total of 6 to 10 mg is given daily. The total daily dose will depend upon what is found to be the optimal level. Many patients derive maximum benefit from this daily total of 6 to 10 mg, but some patients, chiefly those in the postencephalitic group, may require a total daily dose of 12 to 15 mg.
Drug-Induced Parkinsonism
It is sometimes possible to maintain the patient on a reduced trihexyphenidyl HCl dosage after the reactions have remained under control for several days. Instances have been reported in which these reactions have remained in remission for long periods after trihexyphenidyl HCl therapy was discontinued.
Concomitant Use with Levodopa
When trihexyphenidyl HCl is used concomitantly with levodopa, the usual dose of each may need to be reduced. Careful adjustment is necessary, depending on side effects and degree of symptom control. An trihexyphenidyl HCl dosage of 3 to 6 mg daily, in divided doses, is usually adequate.
Concomitant Use with Other Parasympathetic Inhibitors
Trihexyphenidyl HCl may be substituted, in whole or in part, for other parasympathetic inhibitors. The usual technique is partial substitution initially, with progressive reduction in the other medication as the dose of trihexyphenidyl HCl is increased.
.
Trihexyphenidyl HCl tablets - The total daily intake of trihexyphenidyl HCl tablets is tolerated best if divided into 3 doses and taken at mealtimes. High doses (>10 mg daily) may be divided into 4 parts, with 3 doses administered at mealtimes and the fourth at bedtime.
HOW SUPPLIED
Trihexyphenidyl Hydrochloride Tablets, USP 2 mg are white colored, round debossed with N, T on either side of the score line and '2' on the other side.
Trihexyphenidyl Hydrochloride Tablets, USP 2 mg are available in
Bottle of 100 tablets (NDC 16571-160-10)
Bottle of 1000 tablets (NDC 16571-160-11)
Trihexyphenidyl Hydrochloride Tablets, USP 5 mg are white colored, round debossed with N, T on either side of the score line and '5' on other side.
Trihexyphenidyl Hydrochloride Tablets, USP 5 mg are available in
Bottle of 100 tablets (NDC 16571-161-10)
Bottle of 1000 tablets (NDC 16571-161-11)
Dispense in a tight container with child-resistant closure.
Store at 20°-25°C (68°- 77°F). [See USP controlled room temperature.]
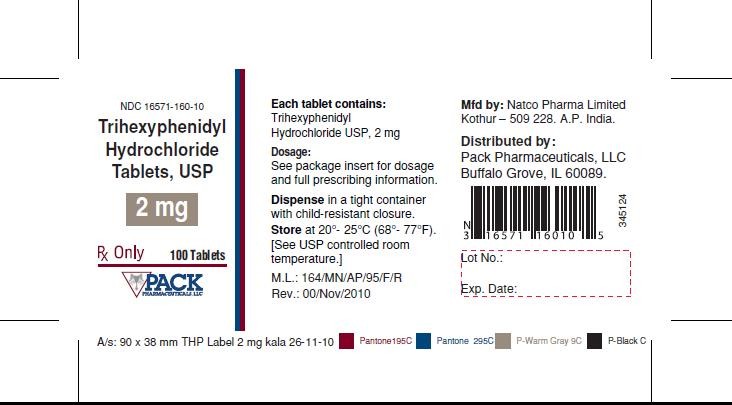
Trihexyphenidyl Hydrochloride Tablets, USP 2 mg-Bottle of 100 tablets
RX Only
NDC 16571-160-10
Each tablet contains: Trihexyphenidyl Hydrochloride USP, 2 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in tight container with child-resistant closure.
Store
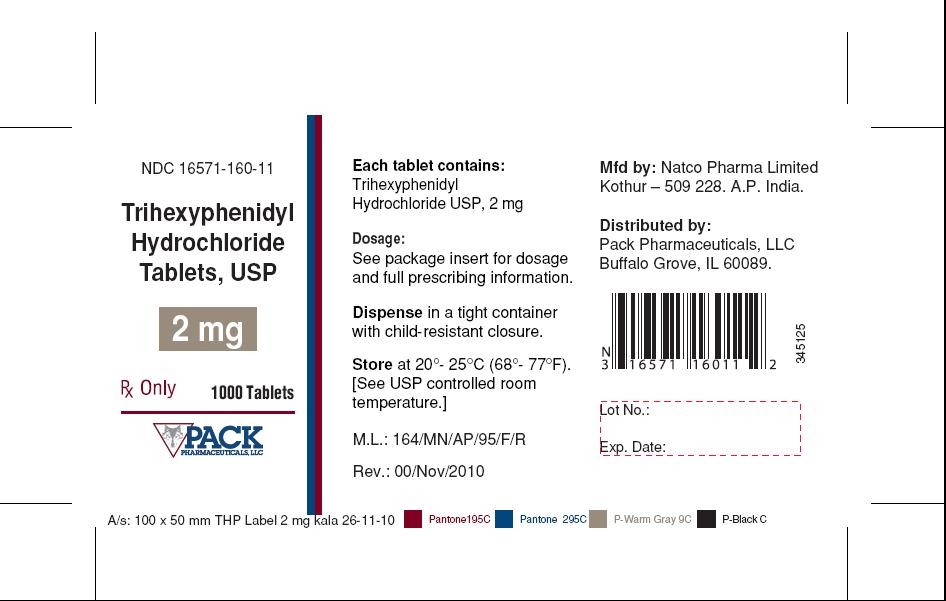
Trihexyphenidyl Hydrochloride Tablets, USP 2 mg-Bottle of 1000 tablets
RX Only
NDC 16571-160-11
Each tablet contains: Trihexyphenidyl Hydrochloride USP, 2 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in tight container with child-resistant closure.
Store
Trihexyphenidyl Hydrochloride Tablets, USP 5 mg-Bottle of 100 tablets
X
Each tablet contains:
Dosage:
Dispense
Store

Trihexyphenidyl Hydrochloride Tablets, USP 5 mg-Bottle of 1000 tablets
RX Only
NDC 16571-161-11
Each tablet contains: Trihexyphenidyl Hydrochloride USP, 5 mg
Dosage: See package insert for dosage and full prescribing information.
Dispense in tight container with child-resistant closure.
Store at 20º-25ºC (68º-77º F).

Trihexyphenidyl HydrochlorideTrihexyphenidyl Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Trihexyphenidyl HydrochlorideTrihexyphenidyl Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||