Tridione
TRIDIONE® (trimethadione)Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRIDIONE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS
- TRIDIONE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TRIDIONE ADVERSE REACTIONS
- OVERDOSAGE
- TRIDIONE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- FDA-APPROVED MEDICATION GUIDE
FULL PRESCRIBING INFORMATION
BECAUSE OF ITS POTENTIAL TO PRODUCE FETAL MALFORMATIONS AND SERIOUS SIDE EFFECTS, TRIDIONE (trimethadione) SHOULD ONLY BE UTILIZED WHEN OTHER LESS TOXIC DRUGS HAVE BEEN FOUND INEFFECTIVE IN CONTROLLING PETIT MAL SEIZURES.
TRIDIONE DESCRIPTION
TRIDIONE (trimethadione) is an antiepileptic agent. An oxazolidinedione compound, it is chemically identified as 3,5,5-trimethyloxozolidine-2,4-dione, and has the following structural formula:
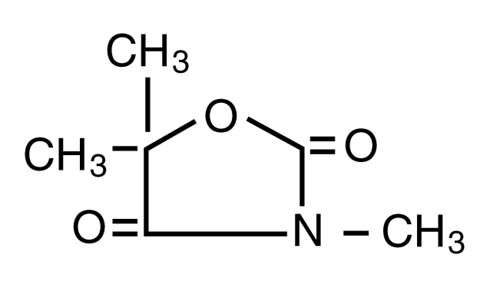
TRIDIONE is a synthetic, water-soluble, white, crystalline powder. It is supplied in tablets for oral use only.
Inactive Ingredients
150 mg Dulcet Tablet: Corn starch, lactose, magnesium stearate, magnesium trisilicate, sucrose and natural/synthetic flavor.
CLINICAL PHARMACOLOGY
TRIDIONE has been shown to prevent pentylenetetrazol-induced and thujone-induced seizures in experimental animals; the drug has a less marked effect on seizures induced by picrotoxin, procaine, cocaine, or strychnine. Unlike the hydantoins and antiepileptic barbiturates, TRIDIONE does not modify the maximal seizure pattern in patients undergoing electroconvulsive therapy.
TRIDIONE has a sedative effect that may increase to the point of ataxia when excessive doses are used. A toxic dose of the drug in animals (approximately 2 g/kg) produced sleep, unconsciousness, and respiratory depression.
Trimethadione is rapidly absorbed from the gastrointestinal tract. It is demethylated by liver microsomes to the active metabolite, dimethadione.
Approximately 3% of a daily dose of TRIDIONE is recovered in the urine as unchanged drug. The majority of trimethadione is excreted slowly by the kidney in the form of dimethadione.
INDICATIONS
TRIDIONE (trimethadione) is indicated for the control of petit mal seizures that are refractory to treatment with other drugs.
TRIDIONE CONTRAINDICATIONS
TRIDIONE is contraindicated in patients with a known hypersensitivity to the drug.
WARNINGS
TRIDIONE may cause serious side effects. Strict medical supervision of the patient is mandatory, especially during the initial year of therapy.
Rash
TRIDIONE (trimethadione) should be withdrawn promptly if skin rash appears, because of the grave possibility of the occurrence of exfoliative dermatitis or severe forms of erythema multiforme. Even a minor acneiform or morbilliform rash should be allowed to clear completely before treatment with TRIDIONE is resumed; reinstitute therapy cautiously.
Blood Dyscrasias
A complete blood count should be done prior to initiating therapy with TRIDIONE, and at monthly intervals thereafter. A marked depression of the blood count is an indication for withdrawal of the drug. If no abnormality appears within 12 months, the interval between blood counts may be extended. A moderate degree of neutropenia with or without a corresponding drop in the leukocyte count is not uncommon. Therapy need not be withdrawn unless the neutrophil count is 2500 or less; more frequent blood examinations should be done when the count is less than 3,000. Other blood dyscrasias, including leukopenia, eosinophilia, thrombocytopenia, pancytopenia, agranulocytosis, hypoplastic anemia, and fatal aplastic anemia, have occurred. Patients should be advised to report immediately such signs and symptoms as sore throat, fever, malaise, easy bruising, petechiae, or epistaxis, or others that may be indicative of an infection or bleeding tendency. TRIDIONE should ordinarily not be used in patients with severe blood dyscrasias.
Liver Dysfunction
Liver function tests should be done prior to initiating therapy with TRIDIONE, and at monthly intervals thereafter. Hepatitis has been reported rarely. Jaundice or other signs of liver dysfunction are an indication for withdrawal of the drug. TRIDIONE should ordinarily not be used in patients with severe hepatic impairment.
Renal Dysfunction
A urinalysis should be done prior to initiating therapy with TRIDIONE and at monthly intervals thereafter. Fatal nephrosis has been reported. Persistent or increasing albuminuria, or the development of any other significant renal abnormality, is an indication for withdrawal of the drug. TRIDIONE should ordinarily not be used in patients with severe renal dysfunction.
Ocular Dysfunction
Hemeralopia has occurred; this appears to be an effect of TRIDIONE on the neural layers of the retina, and usually can be reversed by a reduction in dosage. Scotomata are an indication for withdrawal of the drug. Caution should be observed when treating patients who have diseases of the retina or optic nerve.
Lupus- and Myasthenia-like Syndromes
Manifestations of systemic lupus erythematosus have been associated with the use of TRIDIONE, as they have with the use of certain other anticonvulsants. Lymphadenopathies simulating malignant lymphoma have occurred. Lupus-like manifestations or lymph node enlargement are indications for withdrawal of the drug. Signs and symptoms may disappear after discontinuation of therapy, and specific treatment may be unnecessary.
A myasthenia gravis-like syndrome has been associated with the chronic use of trimethadione. Symptoms suggestive of this condition are indications for withdrawal of the drug.
Drugs known to cause toxic effects similar to those of TRIDIONE should be avoided or used only with extreme caution during therapy with TRIDIONE.
Usage During Pregnancy and Lactation
THERE ARE MULTIPLE REPORTS IN THE CLINICAL LITERATURE WHICH INDICATE THAT THE USE OF ANTICONVULSANT DRUGS DURING PREGNANCY RESULTS IN AN INCREASED INCIDENCE OF BIRTH DEFECTS IN THE OFFSPRING. DATA ARE MORE EXTENSIVE WITH RESPECT TO TRIMETHADIONE, PARAMETHADIONE, PHENYTOIN AND PHENOBARBITAL THAN WITH OTHER ANTICONVULSANT DRUGS.
THEREFORE, ANTICONVULSANT DRUGS SUCH AS TRIDIONE (TRIMETHADIONE) SHOULD BE ADMINISTERED TO WOMEN OF CHILDBEARING POTENTIAL ONLY IF THEY ARE CLEARLY SHOWN TO BE ESSENTIAL IN THE MANAGEMENT OF THEIR SEIZURES. EFFECTIVE MEANS OF CONTRACEPTION SHOULD ACCOMPANY THE USE OF TRIDIONE IN SUCH PATIENTS. IF A PATIENT BECOMES PREGNANT WHILE TAKING TRIDIONE, TERMINATION OF THE PREGNANCY SHOULD BE CONSIDERED. A PATIENT WHO REQUIRES THERAPY WITH TRIDIONE AND WHO WISHES TO BECOME PREGNANT SHOULD BE ADVISED OF THE RISKS.
REPORTS HAVE SUGGESTED THAT THE MATERNAL INGESTION OF ANTICONVULSANT DRUGS, PARTICULARLY BARBITURATES, IS ASSOCIATED WITH A NEONATAL COAGULATION DEFECT THAT MAY CAUSE BLEEDING DURING THE EARLY (USUALLY WITHIN 24 HOURS OF BIRTH) NEONATAL PERIOD. THE POSSIBILITY OF THE OCCURRENCE OF THIS DEFECT WITH THE USE OF TRIDIONE SHOULD BE KEPT IN MIND. THE DEFECT IS CHARACTERIZED BY DECREASED LEVELS OF VITAMIN K-DEPENDENT CLOTTING FACTORS, AND PROLONGATION OF EITHER THE PROTHROMBIN TIME OR THE PARTIAL THROMBOPLASTIN TIME, OR BOTH. IT HAS BEEN SUGGESTED THAT PROPHYLACTIC VITAMIN K BE GIVEN TO THE MOTHER ONE MONTH PRIOR TO, AND DURING DELIVERY, AND TO THE INFANT, INTRAVENOUSLY, IMMEDIATELY AFTER BIRTH.
THE SAFETY OF TRIDIONE FOR USE DURING LACTATION HAS NOT BEEN ESTABLISHED.
To provide information regarding the effects of in utero exposure to Tridione, physicians are advised to recommend that pregnant patients taking Tridione enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including Tridione, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 1 shows absolute and relative risk by indication for all evaluated AEDs.
| Indication | Placebo Patients with Events Per 1000 Patients | Drug Patients with Events Per 1000 Patients | Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients | Risk Difference: Additional Drug Patients with Events Per 1000 Patients |
| Epilepsy | 1.0 | 3.4 | 3.5 | 2.4 |
| Psychiatric | 5.7 | 8.5 | 1.5 | 2.9 |
| Other | 1.0 | 1.8 | 1.9 | 0.9 |
| Total | 2.4 | 4.3 | 1.8 | 1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing Tridione or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
PRECAUTIONS
Abrupt discontinuation of TRIDIONE may precipitate petit mal status. TRIDIONE should always be withdrawn gradually unless serious adverse effects dictate otherwise. In the latter case, another anticonvulsant may be substituted to protect the patient.
Usage During Pregnancy and Lactation
See WARNINGS .
Information for Patients
Physicians should inform patients and their caregivers of the availability of a Medication Guide, and they should be instructed to read the Medication Guide prior to taking Tridione. Patients should be instructed to take Tridione only as prescribed.
Suicidal Thinking and Behavior
Patients, their caregivers, and families should be counseled that AEDs, including Tridione, may increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to the healthcare providers.
Pregnancy Registry
Patients should be encouraged to enroll in the NAAED Pregnancy Registry if they become pregnant. This registry is collecting information about the safety of antiepileptic drugs during pregnancy. To enroll, patients can call the toll free number 1-888-233-2334 (see WARNINGS ).
TRIDIONE ADVERSE REACTIONS
The following side effects, some of them serious, have been associated with the use of TRIDIONE (trimethadione).
Gastrointestinal
Nausea, vomiting, abdominal pain, gastric distress.
CNS/Neurologic
Drowsiness, fatigue, malaise, insomnia, vertigo, headache, paresthesias, precipitation of grand mal seizures, increased irritability, personality changes.
Drowsiness usually subsides with continued therapy. If it persists, a reduction in dosage is indicated.
Hematologic
Bleeding gums, epistaxis, retinal and petechial hemorrhages, vaginal bleeding, neutropenia, leukopenia, eosinophilia, thrombocytopenia, pancytopenia, agranulocytosis, hypoplastic anemia, and fatal aplastic anemia.
Dermatologic
Acneiform or morbilliform skin rash that may progress to exfoliative dermatitis or to severe forms of erythema multiforme.
Other
Hiccups, anorexia, weight loss, hair loss, changes in blood pressure, albuminuria, hemeralopia, photophobia, diplopia.
Fatal nephrosis has occurred.
Hepatitis has been reported rarely.
Lupus erythematosus, and lymphadenopathies simulating malignant lymphoma, have been reported.
Pruritus associated with lymphadenopathy and hepatosplenomegaly has occurred in hypersensitive individuals.
A myasthenia gravis-like syndrome has been reported.
OVERDOSAGE
Symptoms of acute TRIDIONE overdosage include drowsiness, nausea, dizziness, ataxia, visual disturbances. Coma may follow massive overdosage.
Gastric evacuation, either by induced emesis, or by lavage, or both, should be done immediately. General supportive care, including frequent monitoring of the vital signs and close observations of the patient, are required.
Alkalinization of the urine has been reported to enhance the renal excretion of dimethadione, the active metabolite of TRIDIONE.
A blood count and a careful evaluation of hepatic and renal function should be done following recovery.
TRIDIONE DOSAGE AND ADMINISTRATION
TRIDIONE is administered orally.
Usual Adult Dosage
0.9-2.4 grams daily in 3 or 4 equally divided doses (i.e., 300−600 mg 3 or 4 times daily).
Initially, give 0.9 gram daily; increase this dose by 300 mg at weekly intervals until therapeutic results are seen or until toxic symptoms appear.
Maintenance dosage should be the least amount of drug required to maintain control.
Children's Dosage
Usually 0.3-0.9 gram daily in 3 or 4 equally divided doses.
HOW SUPPLIED
TRIDIONE Dulcet® Tablets (trimethadione tablets) are supplied as white, chewable tablets bearing the “a” logo and a two-letter code designation LE for the 150 mg tablet, in bottles of 100 (NDC 0074-3753-01).
Recommended Storage
Store Dulcet tablets in refrigerator 36° to 46°F (2° - 8°C) to minimize crystallization. However some crystallization not harmful to product may occur. Keep tightly closed.
Dulcet® sweetened tablets, AbbVie Inc.
AbbVie Inc.
North Chicago, IL 60064, U.S.A.
FDA-APPROVED MEDICATION GUIDE
MEDICATION GUIDE
TRIDIONE (trI-'dI-"On)
(trimethadione)
Tablets
Read this Medication Guide before you start taking TRIDIONE and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about TRIDIONE?
Do not stop taking TRIDIONE without first talking to your healthcare provider.
Stopping TRIDIONE suddenly can cause serious problems.
TRIDIONE can cause serious side effects, including:
-
Rash. This may need to be treated in a hospital and may be life-threatening.
Call your healthcare provider right away if you have any of these symptoms:- skin rash
- hives
- sores in your mouth
-
Blood problems that can be life-threatening. Call your healthcare provider right away if you have any of these symptoms:
- Fever, swollen glands, or sore throat that come and go or do not go away
- Frequent infections or an infection that does not go away
- Easy bruising
- Red or purple spots on your body
- Bleeding gums or nose bleeds
- Severe fatigue or weakness
-
Liver problems. Call your healthcare provider right away if you have any of these symptoms:
- yellowing of your skin or the whites of your eyes (jaundice)
- dark urine
- nausea or vomiting
- loss of appetite
- pain on the right side of your stomach (abdomen)
- Kidney problems that may be life-threatening.
-
Eye problems. Call your healthcare provider right away if you have any new changes in your vision such as:
- problems seeing in bright light
- blurred vision
-
Birth defects in your unborn baby.
- Women who can become pregnant should talk to their healthcare provider about using other possible treatments instead of TRIDIONE. If the decision is made to use TRIDIONE, women should use effective birth control (contraception). Talk with your healthcare provider if you are pregnant or plan to become pregnant. Birth defects may occur even in children born to women who are not taking any medicines and do not have other risk factors.
- Tell your healthcare provider right away if you become pregnant while taking TRIDIONE. You and your healthcare provider should decide if you will continue to take TRIDIONE while you are pregnant.
- If you become pregnant while taking TRIDIONE, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic drugs during pregnancy.
- Tell your healthcare provider right away if you are breastfeeding or plan to breastfeed. It is unknown if TRIDIONE passes into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take TRIDIONE.
-
Like other antiepileptic drugs, TRIDIONE may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
- Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop TRIDIONE without first talking to a healthcare provider. Stopping TRIDIONE suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
What is TRIDIONE?
TRIDIONE is a prescription medicine used to treat absence (petit mal) seizures that are not controlled with other drugs.
Who should not take TRIDIONE?
Do not take TRIDIONE if you are allergic to trimethadione or any of the ingredients in TRIDIONE. See the end of this leaflet for a complete list of ingredients in TRIDIONE.
What should I tell my healthcare provider before taking TRIDIONE?
Before you take TRIDIONE, tell your healthcare provider if you have or have had:
- blood problems
- kidney problems
- liver problems
- eye problems
- depression, mood problems or suicidal thoughts or behavior
- any other medical conditions
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I take TRIDIONE?
- Take TRIDIONE exactly as your healthcare provider tells you.
- TRIDIONE can be chewed or swallowed whole.
- Your healthcare provider may change your dose of TRIDIONE. Do not change your dose of TRIDIONE without talking to your healthcare provider.
- If you take too much TRIDIONE, call your healthcare provider or local Poison Control Center right away.
- Do not stop taking TRIDIONE without talking to your healthcare provider. Stopping TRIDIONE suddenly can cause serious problems, including seizures that will not stop (status epilepticus).
What should I avoid while taking TRIDIONE?
- Do not drink alcohol or take other drugs that make you sleepy or dizzy while taking TRIDIONE until you talk to your healthcare provider. TRIDIONE taken with alcohol or drugs that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how TRIDIONE affects you. TRIDIONE can slow your thinking and motor skills.
What are the possible side effects of TRIDIONE?
See “What is the most important information I should know about TRIDIONE?”
TRIDIONE may cause other serious side effects, including:
-
Symptoms that are like the symptoms of lupus or myasthenia gravis. Call your healthcare provider right away if you have any of these symptoms:
- droopy eyelids
- a rash on your cheeks or other parts of your body
- sensitivity to the sun
- new joint or muscle pains
- chest pain or shortness of breath
- swelling of your feet, ankles, and legs
- weakness of your arms or legs
- problems swallowing
- speech problems
- swollen glands (enlarged lymph nodes)
The most common side effects of TRIDIONE include:
- nausea
- sleepiness
- tiredness
- increase in seizures
- feelings of anger and frustration
- changes in behavior
These are not all the possible side effects of TRIDIONE. For more information, ask your healthcare provider or pharmacist.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store TRIDIONE?
- Store TRIDIONE in the refrigerator at 36 to 460F (2 to 80C).
- Keep TRIDIONE in a tightly closed container.
Keep TRIDIONE and all medicines out of the reach of children.
General Information about TRIDIONE
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use TRIDIONE for a condition for which it was not prescribed. Do not give TRIDIONE to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about TRIDIONE. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about TRIDIONE that is written for health professionals. For more information, go to www.rxabbvie.com or call 1-800-633-9110.
What are the ingredients in TRIDIONE?
Active ingredient: trimethadione
Inactive ingredient: corn starch, lactose, magnesium stearate, magnesium trisilicate, sucrose and natural/synthetic flavor
Dulcet sweetened tablets, AbbVie Inc.
AbbVie Inc.
North Chicago, IL 60064, U.S.A.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
03-A773 April, 2013
NDC 0074–3753–01
100 Tablets Dulcet®
TRIDIONE®
TRIMETHADIONE TABLETS 150 mg (2 1/2 grs)
Rx only abbvie

TridioneTrimethadione TABLET, CHEWABLE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||