Tramadol Hydrochloride
Rhodes Pharmaceuticals L.P.
Rhodes Pharmaceuticals L.P.
Tramodol Hydrochloride Extended-Release Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- TRAMADOL HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- TRAMADOL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- TRAMADOL HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- TRAMADOL HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
TRAMADOL HYDROCHLORIDE DESCRIPTION
Tramadol Hydrochloride Extended-Release Tablets are a centrally acting analgesic composed of a dual-matrix delivery system with both immediate-release and extended-release characteristics. The chemical name for tramadol hydrochloride is (±)cis-2-[(dimethylamino) methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride. Its structural formula is:
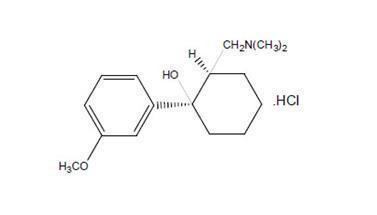
C16H25NO2 ·HCl
The molecular weight of tramadol hydrochloride is 299.8. Tramadol hydrochloride is a white crystalline powder that is freely soluble in water and ethanol. Tramadol Hydrochloride Extended-Release Tablets are for oral administration and contain 100 mg, 200 mg or 300 mg of tramadol hydrochloride. The tablets are white to off-white in color. The inactive ingredients in the tablet are colloidal silicon dioxide, pregelatinized modified starch, hydrogenated vegetable oil, magnesium stearate, polyvinyl acetate, povidone, sodium lauryl sulfate and xanthan gum.
CLINICAL PHARMACOLOGY
Mechanism of Action
Tramadol Hydrochloride Extended-Release Tablets are a centrally acting synthetic opioid analgesic. Although its mode of action is not completely understood, at least two complementary mechanisms that demonstrate three different types of activity appear applicable: binding of parent and M1 metabolite to µ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.
Opioid activity is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite (M1) to mu-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in mu-opioid binding. Tramadol-induced analgesia is only partially antagonized by the opiate antagonist naloxone in several animal tests. The relative contribution of both tramadol and M1 to human analgesia is dependent upon the plasma concentrations of each compound.
Tramadol has been shown to inhibit reuptake of norepinephrine and serotonin in vitro, as have some other opioid analgesics. These mechanisms may contribute independently to the overall analgesic profile of tramadol.
Apart from analgesia, tramadol hydrochloride administration may produce various symptoms (including dizziness, somnolence, nausea, constipation, sweating and pruritus) similar to that of other opioids. In contrast to morphine, tramadol has not been shown to cause histamine release. At therapeutic doses, tramadol has no effect on heart rate, left-ventricular function or cardiac index. Orthostatic hypotension has been observed.
Pharmacokinetics
The analgesic activity of tramadol hydrochloride is due to both parent drug and the M1 metabolite (see CLINICAL PHARMACOLOGY, Mechanism of Action).
Tramadol Hydrochloride Extended-Release Tablets are formulated as a racemate and both tramadol and M1 are detected in the circulation.
The pharmacokinetics of tramadol and M1 are dose-proportional over a 100 to 300 mg dose range in healthy subjects.
The median time to peak plasma concentrations of tramadol and M1 after multiple-dose administration of Tramadol Hydrochloride Extended-Release 200 mg tablets to healthy subjects are attained at about 4 h and 5 h, respectively (Table 1 and Figure 1).
The pharmacokinetic parameter values for Tramadol Hydrochloride Extended-Release Tablets 200 mg administered once daily and tramadol immediate-release 50 mg administered every six hours are provided in Table 1. The relative bioavailability of a 200 mg Tramadol Hydrochloride Extended-Release Tablet compared to a 50 mg immediate-release tablet dosed every six hours was approximately 95% in healthy subjects.
| *Tmax is presented as Median (Range) | ||||
|
Pharmacokinetic Parameter |
Tramadol | M1 Metabolite | ||
| Tramadol Hydrochloride Extended-release 200 mg Tablet Once-Daily |
Immediate- release tramadol 50 mg Tablet Every 6 Hours |
Tramadol Hydrochloride Extended-release 200 mg Tablet Once-Daily |
Immediate- release tramadol 50 mg Tablet Every 6 Hours |
|
| AUC0-24 (ng∙h/mL) | 5991 (22) | 6399 (28) | 1361 (27) | 1438 (23) |
| Cmax (ng/mL) | 345 (21) | 423 (23) | 71 (27) | 79 (22) |
| Cmin (ng/mL) | 157 (31) | 190 (34) | 41 (30) | 50 (29) |
| Tmax (hr)* | 4.0 (3.0 – 9.0) | 1.0 (1.0 – 3.0) | 5.0 (3.0 – 20) | 1.5 (1.0 – 3.0) |
| Fluctuation (%) | 77 (26) | 91 (22) | 53 (29) | 49 (26) |
Steady-state plasma concentrations are reached within approximately 48 hours.
Figure 1. Mean Tramadol Plasma Concentrations at Steady State Following Five Days of Oral Administration of Tramadol Hydrochloride Extended-Release Tablets 200 mg Once Daily and Immediate-Release Tramadol 50 mg Every 6 Hours.
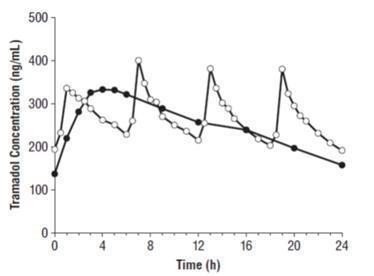
‒●‒ Tramadol Hydrochloride Extended-Release Tablets, 200 mg q.d.
‒ᴼ‒ Immediate-Release Tramadol Tablets, 50 mg q6h
Figure 2. Mean M1 Plasma Concentrations at Steady State Following Five Days of Oral Administration of Tramadol Hydrochloride Extended-Release Tablets 200 mg Once Daily and Immediate-Release Tramadol 50 mg Every 6 Hours.
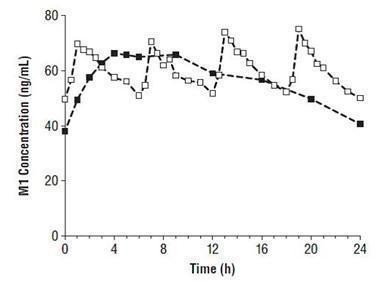
--■-- Tramadol Hydrochloride Extended-Release Tablets, 200 mg q.d.
--□-- Immediate-Release Tramadol Tablets, 50 mg q6h
Food Effect
Co-administration with a high fat meal did not significantly affect AUC (overall exposure to tramadol); however, Cmax (peak plasma concentration) increased 67% following a single 300 mg tablet administration and 54% following a single 200 mg tablet administration. Tramadol Hydrochloride Extended-Release Tablets was administered without regard to food in all clinical trials.
The volume of distribution of tramadol is 2.6 and 2.9 L/kg in males and females, respectively, following a 100 mg intravenous dose. The binding of tramadol to human plasma proteins is approximately 20%. Protein binding also appears to be independent of concentration up to 10 µg/mL. Saturation of plasma protein binding occurs only at concentrations outside the clinically relevant range.
Tramadol is extensively metabolized after oral administration. The major metabolic pathways appear to be N- and O-demethylation and glucuronidation or sulfation in the liver. N-demethylation is mediated by CYP3A4 and CYP2B6. One metabolite (O-desmethyltramadol, denoted M1) is pharmacologically active in animal models. Formation of M1 is dependent on CYP2D6 and as such is subject to inhibition and polymorphism, which may affect the therapeutic response (see PRECAUTIONS, Drug Interactions).
Tramadol is eliminated primarily through metabolism by the liver and the metabolites are eliminated primarily by the kidneys. Approximately 30% of the dose is excreted in the urine as unchanged drug, whereas 60% of the dose is excreted as metabolites. The remainder is excreted either as unidentified or as unextractable metabolites. After single administration of Tramadol Hydrochloride Extended-Release Tablets, the mean terminal plasma elimination half-lives of racemic tramadol and racemic M1 are 6.5 ± 1.5 and 7.5 ± 1.4 hours, respectively.
Impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1 in patients taking an immediate-release formulation of tramadol. Tramadol Hydrochloride Extended-Release Tablets has not been studied in patients with renal impairment. The limited availability of dose strengths and once daily dosing of Tramadol Hydrochloride Extended-Release Tablets do not permit the dosing flexibility required for safe use in patients with severe renal impairment. Therefore, Tramadol Hydrochloride Extended-Release Tablets should not be used in patients with severe renal impairment (creatinine clearance less than 30 mL/min) (see PRECAUTIONS, Use in Renal and Hepatic Disease and DOSAGE AND ADMINISTRATION). The total amount of tramadol and M1 removed during a 4-hour dialysis period is less than 7% of the administered dose.
The metabolism of tramadol and M1 is reduced in patients with advanced cirrhosis of the liver, resulting in both a larger area under the concentration time curve (AUC) for tramadol and longer mean tramadol and M1 elimination half-lives (13 hours for tramadol and 19 hours for M1) after the administration of tramadol immediate-release tablets. Tramadol Hydrochloride Extended-Release Tablets has not been studied in patients with hepatic impairment. The limited availability of dose strengths and once daily dosing of Tramadol Hydrochloride Extended-Release Tablets do not permit the dosing flexibility required for safe use in patients with hepatic impairment. Therefore, Tramadol Hydrochloride Extended-Release Tablets should not be used in patients with hepatic impairment (see PRECAUTIONS, Use in Renal and Hepatic Disease and DOSAGE AND ADMINISTRATION).
Healthy elderly subjects aged 65 to 75 years administered an immediate-release formulation of tramadol, have plasma concentrations and elimination half-lives comparable to those observed in healthy subjects less than 65 years of age. In subjects over 75 years, mean maximum plasma concentrations are elevated (208 vs. 162 ng/mL) and the mean elimination half-life is prolonged (7 vs. 6 hours) compared to subjects 65 to 75 years of age. Adjustment of the daily dose is recommended for patients older than 75 years (see DOSAGE AND ADMINISTRATION).
Following a 100 mg IV dose of tramadol, plasma clearance was 6.4 mL/min/kg in males and 5.7 mL/min/kg in females. Following a single oral dose of immediate-release tramadol, and after adjusting for body weight, females had a 12% higher peak tramadol concentration and a 35% higher area under the concentration-time curve compared to males. The clinical significance of this difference is unknown.
The formation of the active metabolite of tramadol, M1, is mediated by CYP2D6, a polymorphic enzyme. Approximately 7% of the population has reduced activity of CYP2D6. These individuals are "poor metabolizers" of debrisoquine, dextromethorphan and tricyclic antidepressants, among other drugs. In studies in healthy subjects administered immediate-release tramadol products, concentrations of tramadol were approximately 20% higher in "poor metabolizers" versus "extensive metabolizers", while M1 concentrations were 40% lower. In vitro drug interaction studies in human liver microsomes indicate that inhibitors of CYP2D6 (amitriptyline, quinidine and fluoxetine and its metabolite norfluoxetine,) inhibit the metabolism of tramadol to various degrees, suggesting that concomitant administration of these compounds could result in increases in tramadol concentrations and decreased concentrations of M1. The full pharmacological impact of these alterations in terms of either efficacy or safety is unknown.
Tramadol is also metabolized by CYP3A4. Administration of CYP3A4 inhibitors, such as ketoconazole and erythromycin, or inducers, such as rifampin and St. John’s Wort, with Tramadol Hydrochloride Extended-Release Tablets may affect the metabolism of tramadol leading to altered tramadol exposure (see PRECAUTIONS).
Quinidine
Quinidine is a selective inhibitor of CYP2D6, so that concomitant administration of quinidine and Tramadol Hydrochloride Extended-Release Tablets may result in increased concentrations of tramadol and reduced concentrations of M1. The clinical consequences of these findings are unknown (see PRECAUTIONS). In vitro drug interaction studies in human liver microsomes indicate that tramadol has no effect on quinidine metabolism.
Carbamazepine
Carbamazepine, a CYP3A4 inducer, increases tramadol metabolism. Patients taking carbamazepine may have a significantly reduced analgesic effect of tramadol. Because of the seizure risk associated with tramadol, concomitant administration of Tramadol Hydrochloride Extended-Release Tablets and carbamazepine is not recommended (see PRECAUTIONS).
Cimetidine
Concomitant administration of tramadol immediate-release tablets with cimetidine does not result in clinically significant changes in tramadol pharmacokinetics. No alteration of the Tramadol Hydrochloride Extended-Release Tablets dosage regimen with cimetidine is recommended.
Clinical Studies
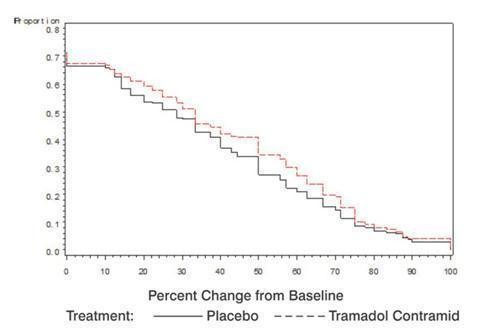
Tramadol Hydrochloride Extended-Release Tablets were studied in four 12-week, randomized, double-blind, controlled studies in patients with moderate to severe pain due to osteoarthritis. Efficacy was demonstrated in one double-blind, placebo-controlled, randomized withdrawal design study. In this study, patients who experienced a reduction of pain and were able to tolerate Tramadol Hydrochloride Extended-Release Tablets during an open-label titration period, were then randomized to Tramadol Hydrochloride Extended-Release Tablets or to placebo for 12 weeks. Sixty-five percent of patients were able to successfully titrate onto Tramadol Hydrochloride Extended-Release Tablets. After a washout, patients randomized to Tramadol Hydrochloride Extended-Release Tablets were titrated to 200 mg or 300 mg of Tramadol Hydrochloride Extended-Release Tablets based on tolerability and remained on that dose for the following 12-week period. Approximately 24% of patients discontinued during the randomized period of the study, with more patients discontinuing from the Tramadol Hydrochloride Extended-Release Tablets arm than the placebo arm due to adverse events (10% vs. 5%, respectively) and more patients discontinuing from the placebo arm than the Tramadol Hydrochloride Extended-Release Tablets arm due to lack of efficacy (10% vs. 8%, respectively). Patients treated with Tramadol Hydrochloride Extended-Release Tablets demonstrated a greater improvement in pain intensity, measured on an 11-point numerical rating scale, at the end of treatment compared to patients randomized to placebo. Figure 3 shows the fraction of patients achieving various degrees of improvement in pain from baseline to the end of treatment (week 12). The figure is cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned 0% improvement.
Figure 3. Proportion of Patients Achieving Various Levels of Pain Relief as Measured by 12-Week Pain Intensity.
 INDICATIONS AND USAGE
INDICATIONS AND USAGE
Tramadol Hydrochloride Extended-Release Tablets are indicated for the management of moderate to moderately severe chronic pain in adults who require around-the-clock treatment of their pain for an extended period of time.
TRAMADOL HYDROCHLORIDE CONTRAINDICATIONS
Tramadol Hydrochloride Extended-Release Tablets should not be administered to patients who have previously demonstrated hypersensitivity to tramadol, any other component of this product or opioids.
Tramadol Hydrochloride Extended-Release Tablets are contraindicated in patients with significant respiratory depression in unmonitored settings or the absence of resuscitative equipment. Tramadol Hydrochloride Extended-Release Tablets are also contraindicated in patients with acute or severe bronchial asthma or hypercapnia in unmonitored settings or the absence of resuscitative equipment.
WARNINGS
Seizure Risk
Seizures have been reported in patients receiving tramadol hydrochloride within the recommended dosage range. Spontaneous postmarketing reports indicate that seizure risk is increased with doses above the recommended range. Concomitant use of tramadol hydrochloride increases the seizure risk in patients taking:
- Selective serotonin reuptake inhibitors (SSRI antidepressants or anorectics),
- Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), or
- Other opioids.
Administration of Tramadol Hydrochloride Extended-Release Tablets may enhance the seizure risk in patients taking:
- Monoamine Oxidase (MAO) inhibitors (see WARNINGS, Use with MAO Inhibitors and Serotonin Re-uptake Inhibitors),
- Neuroleptics, or
- Other drugs that reduce the seizure threshold.
Risk of convulsions may also be increased in patients with epilepsy, those with a history of seizures, or in patients with a recognized risk for seizure (such as head trauma, certain metabolic disorders, alcohol and drug withdrawal and CNS infections). In tramadol overdose, naloxone administration may increase the risk of seizures.
Suicide Risk
Do not prescribe Tramadol Hydrochloride Extended-Release Tablets for patients who are suicidal or addiction-prone. Prescribe Tramadol Hydrochloride Extended-Release Tablets with caution for patients taking tranquilizers or antidepressant drugs and for patients who use alcohol in excess. Serious potential consequences of overdosage with Tramadol Hydrochloride Extended-Release Tablets are central nervous system depression, respiratory depression and death. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment (see OVERDOSAGE).
Serotonin Syndrome Risk
The development of a potentially life-threatening serotonin syndrome may occur with the use of tramadol products, including Tramadol Hydrochloride Extended-Release Tablets, particularly with concomitant use of serotonergic drugs such as SSRIs, SNRIs, TCAs, MAOIs, and triptans, with drugs which impair metabolism of serotonin (including MAOIs), and with drugs which impair metabolism of tramadol (CYP2D6 and CYP3A4 inhibitors). This may occur within the recommended dose (see CLINICAL PHARMACOLOGY, Pharmacokinetics).
Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Tramadol products in excessive doses, either alone or in combination with other Central Nervous System (CNS) depressants, including alcohol, are a major cause of drug-related deaths. Fatalities within the first hour of overdosage are not uncommon. Tramadol should not be taken in doses higher than those recommended by the physician. The judicious prescribing of tramadol is essential to the safe use of this drug. With patients who are depressed or suicidal, consideration should be given to the use of non-narcotic analgesics. Patients should be cautioned about the concomitant use of tramadol products and alcohol because of potentially serious CNS-additive effects of these agents. Because of its added depressant effects, tramadol should be prescribed with caution for those patients whose medical condition requires the concomitant administration of sedatives, tranquilizers, muscle relaxants, antidepressants, or other CNS-depressant drugs. Patients should be advised of the additive depressant effects of these combinations.
Many of the tramadol-related deaths have occurred in patients with previous histories of emotional disturbances or suicidal ideation or attempts as well as histories of misuse of tranquilizers, alcohol, and other CNS-active drugs. Some deaths have occurred as a consequence of the accidental ingestion of excessive quantities of tramadol alone or in combination with other drugs. Patients taking tramadol should be warned not to exceed the dose recommended by their physician.
Serious and rarely fatal anaphylactoid reactions have been reported in patients receiving therapy with tramadol. When these events do occur, it is often following the first dose. Other reported allergic reactions include pruritus, hives, bronchospasm, angioedema, toxic epidermal necrolysis and Stevens-Johnson syndrome. Patients with a history of anaphylactoid reactions to other opioids may be at increased risk and therefore should not receive Tramadol Hydrochloride Extended-Release Tablets (see CONTRAINDICATIONS).
Tramadol Hydrochloride Extended-Release Tablets should be administered cautiously in patients at risk for respiratory depression. In these patients, alternative non-opioid analgesics should be considered. When large doses of tramadol are administered with anesthetic medications or alcohol, respiratory depression may result. Respiratory depression should be treated as an overdose. If naloxone is to be administered, use cautiously because it may precipitate seizures (see WARNINGS, Seizure Risk and OVERDOSAGE).
Tramadol Hydrochloride Extended-Release Tablets should be used with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics. Tramadol increases the risk of CNS and respiratory depression in these patients.
Tramadol Hydrochloride Extended-Release Tablets should be used with caution in patients with increased intracranial pressure or head injury. The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, and may be markedly exaggerated in these patients. Additionally, pupillary changes (miosis) from tramadol may obscure the existence, extent, or course of intracranial pathology. Clinicians should also maintain a high index of suspicion for adverse drug reaction when evaluating altered mental status in these patients if they are receiving Tramadol Hydrochloride Extended-Release Tablets (see WARNINGS, Respiratory Depression).
Tramadol Hydrochloride Extended-Release Tablets may impair the mental and physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Patients using this drug should be cautioned accordingly.
Tramadol Hydrochloride Extended-Release Tablets should be used with great caution in patients taking MAO inhibitors. Animal studies have shown increased deaths with combined administration of tramadol and MAO inhibitors. Concomitant use of tramadol products with MAO inhibitors or SSRIs increases the risk of adverse events, including seizure and serotonin syndrome.
Withdrawal symptoms may occur if Tramadol Hydrochloride Extended-Release Tablets are discontinued abruptly. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations.
In a 12 week study, 325 patients were followed for 3 and 7 days after discontinuation of treatment with Tramadol Hydrochloride Extended-Release Tablets. The majority of reported post-treatment adverse events including withdrawal symptoms were mild to moderate in nature. Onset of the post-treatment adverse events occurred more frequently within the first three days after treatment was stopped. Less than 1% of patients taking Tramadol Hydrochloride Extended-Release Tablets met the DSM-IV criteria for a diagnosis of opioid withdrawal.
Clinical experience suggests that signs and symptoms of withdrawal may be reduced by tapering medication when discontinuing tramadol therapy.
Tramadol is an opioid agonist of the morphine type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Like other opioid agonists, legal or illicit, tramadol can be abused. This should be considered when prescribing or dispensing Tramadol Hydrochloride Extended-Release Tablets in situations where the healthcare professional is concerned about a risk of misuse, abuse, or diversion.
Tramadol Hydrochloride Extended-Release Tablets could be abused by breaking, crushing, chewing, or dissolving the product which can result in the uncontrolled delivery of the opioid, and as a consequence poses a significant risk of overdose and death.
Concerns about abuse, addiction, and diversion should not prevent the proper management of pain.
Healthcare professionals should contact their State Professional Licensing Board or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Interactions with Alcohol and Drugs of Abuse
Tramadol may be expected to have additive effects when used in conjunction with alcohol, other opioids or drugs, whether legal or illicit, which cause central nervous system depression.
DRUG ABUSE AND ADDICTION
Abuse
Tramadol Hydrochloride Extended-Release Tablets are a mu-agonist opioid. Tramadol, like other opioids used in analgesia, can be abused and is subject to criminal diversion.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
Concerns about abuse and addiction should not prevent the proper management of pain. However all patients treated with opioids require careful monitoring for signs of abuse and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
“Drug-seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Tramadol Hydrochloride Extended-Release Tablets, like other opioids, may be diverted for non-medical use. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Tramadol Hydrochloride Extended-Release Tablets are intended for oral use only. The crushed tablet poses a hazard of overdose and death. This risk is increased with concurrent abuse of alcohol and other substances. With parenteral abuse, the tablet excipients can be expected to result in local tissue necrosis, infection, pulmonary granulomas, and increased risk of endocarditis and valvular heart injury. Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Physical dependence is manifested by withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an antagonist.
The opioid abstinence or withdrawal syndrome is characterized by some or all of the following: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate. Generally, tolerance and/or withdrawal are more likely to occur the longer a patient is on continuous opioid therapy.
Serious potential consequences of overdosage with Tramadol Hydrochloride Extended-Release Tablets are central nervous system depression, respiratory depression and death. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment (see OVERDOSAGE).
PRECAUTIONS
The administration of Tramadol Hydrochloride Extended-Release Tablets may complicate the clinical assessment of patients with acute abdominal conditions.
Impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1 in patients taking an immediate-release formulation of tramadol. Tramadol Hydrochloride Extended-Release Tablets has not been studied in patients with renal impairment. The limited availability of dose strengths and once daily dosing of Tramadol Hydrochloride Extended-Release Tablets does not permit the dosing flexibility required for safe use in patients with severe renal impairment. Therefore, Tramadol Hydrochloride Extended-Release Tablets should not be used in patients with severe renal impairment (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
The metabolism of tramadol and M1 is reduced in patients with advanced cirrhosis of the liver. Tramadol Hydrochloride Extended-Release Tablets has not been studied in patients with hepatic impairment. The limited availability of dose strengths and once daily dosing of Tramadol Hydrochloride Extended-Release Tablets does not permit the dosing flexibility required for safe use in patients with hepatic impairment. Therefore, Tramadol Hydrochloride Extended-Release Tablets should not be used in patients with hepatic impairment (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Information for Patients
- Tramadol Hydrochloride Extended-Release Tablets are for oral use only and should be swallowed whole with a sufficient quantity of liquid and not split, chewed, dissolved or crushed.
- Tramadol Hydrochloride Extended-Release Tablets may cause seizures and/or serotonin syndrome with concomitant use of serotonergic agents (including SSRIs, SNRIs and triptans) or drugs that significantly reduce the metabolic clearance of tramadol.
- Tramadol Hydrochloride Extended-Release Tablets should be taken once daily, at approximately the same time every day and that exceeding these instructions can result in respiratory depression, seizures or death.
- Tramadol Hydrochloride Extended-Release Tablets should not be taken in doses exceeding the maximum recommended daily dose as exceeding these recommendations can result in respiratory depression, seizures or even death (see DOSAGE AND ADMINISTRATION).
- Tramadol Hydrochloride Extended-Release Tablets may impair the mental and physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. Patients using this drug should be cautioned accordingly.
- Tramadol Hydrochloride Extended-Release Tablets should not be taken with alcohol-containing beverages.
- Tramadol Hydrochloride Extended-Release Tablets should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics.
- Female patients should be instructed to inform the physician if they are pregnant, think they might become pregnant, or are trying to become pregnant (see PRECAUTIONS, Pregnancy, and Labor and Delivery).
- Clinical experience suggests that signs and symptoms of withdrawal may be reduced by tapering medication when discontinuing tramadol therapy.
- Patients should be informed to keep Tramadol Hydrochloride Extended-Release Tablets out of reach of children.
Tramadol Hydrochloride Extended-Release Tablets are an opioid with no approved use for the management of addictive disorders. Its proper usage in individuals with drug or alcohol dependence, either active or in remission is for the management of pain requiring opioid analgesia.
Drug Interactions
CYP2D6 and CYP3A4 Inhibitors: Concomitant administration of CYP2D6 and/or CYP3A4 inhibitors (see CLINICAL PHARMACOLOGY, Pharmacokinetics), such as quinidine, fluoxetine, paroxetine and amitriptyline (CYP2D6 inhibitors), and ketoconazole and erythromycin (CYP3A4 inhibitors), may reduce metabolic clearance of tramadol increasing the risk for serious adverse events including seizures and serotonin syndrome.
Serotonergic Drugs: There have been postmarketing reports of serotonin syndrome with use of tramadol and SSRIs/SNRIs or MAOIs and α2-adrenergic blockers. Caution is advised when Tramadol Hydrochloride Extended-Release Tablets are coadministered with other drugs that may affect the serotonergic neurotransmitter systems, such as SSRIs, MAOIs, triptans, linezolid (an antibiotic which is a reversible non-selective MAOI), lithium, or St. John’s Wort. If concomitant treatment of Tramadol Hydrochloride Extended-Release Tablets with a drug affecting the serotonergic neurotransmitter system is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases (see WARNINGS, Serotonin Syndrome ).
Triptans: Based on the mechanism of action of tramadol and the potential for serotonin syndrome, caution is advised when Tramadol Hydrochloride Extended-Release Tablets are coadministered with a triptan. If concomitant treatment of Tramadol Hydrochloride Extended-Release Tablets with a triptan is clinically warranted, careful observation of the patient is advised, particularly during treatment initiation and dose increases (see WARNINGS, Serotonin Syndrome).
Patients taking carbamazepine, a CYP3A4 inducer, may have a significantly reduced analgesic effect. Because carbamazepine increases tramadol metabolism and because of the seizure risk associated with tramadol, concomitant administration of Tramadol Hydrochloride Extended-Release Tablets and carbamazepine is not recommended.
Tramadol is metabolized to M1 by CYP2D6. Quinidine is a selective inhibitor of that isoenzyme, so that concomitant administration of quinidine and tramadol products results in increased concentrations of tramadol and reduced concentrations of M1. The clinical consequences of these findings are unknown. In vitro drug interaction studies in human liver microsomes indicate that tramadol has no effect on quinidine metabolism.
Post-marketing surveillance of tramadol has revealed rare reports of digoxin toxicity and alteration of warfarin effect, including elevation of prothrombin times.
Interaction With Central Nervous System (CNS) Depressants
Tramadol Hydrochloride Extended-Release Tablets should be used with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics. Tramadol Hydrochloride Extended-Release Tablets increases the risk of CNS and respiratory depression in these patients.
Potential of Other Drugs to Affect Tramadol
In vitro drug interaction studies in human liver microsomes indicate that concomitant administration with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and amitriptyline could result in some inhibition of the metabolism of tramadol.
Tramadol is partially metabolized by CYP3A4. Administration of CYP3A4 inhibitors, such as ketoconazole and erythromycin, or inducers, such as rifampin and St. John’s Wort, with Tramadol Hydrochloride Extended-Release Tablets may affect the metabolism of tramadol leading to altered tramadol exposure.
Potential for Tramadol to Affect Other Drugs
In vitro studies indicate that tramadol is unlikely to inhibit the CYP3A4-mediated metabolism of other drugs when administered concomitantly at therapeutic doses. Tramadol is a mild inducer of selected drug metabolism pathways measured in animals.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A slight, but statistically significant increase in two common murine tumors, pulmonary and hepatic, was observed in a mouse carcinogenicity study, particularly in aged mice. Mice were dosed orally up to 30 mg/kg (90 mg/m2 or 0.5 times the maximum daily human dosage of 185 mg/m2) for approximately two years, although the study was not done with the Maximum Tolerated Dose. This finding is not believed to suggest risk in humans. No such finding occurred in a rat carcinogenicity study (dosing orally up to 30 mg/kg to 180 mg/m2 equal to the maximum daily human dosage of tramadol).
Tramadol was not mutagenic in the following assays: Ames Salmonella microsomal activation test, CHO/HPRT mammalian cell assay, mouse lymphoma assay (in the absence of metabolic activation), dominant lethal mutation tests in mice, chromosome aberration test in Chinese hamsters, and bone marrow micronucleus tests in mice and Chinese hamsters. Positive mutagenic results occurred in the presence of metabolic activation in the mouse lymphoma assay and micronucleus test in rats. Relevance of the finding in humans is unknown.
No effects on fertility were observed for tramadol at oral dose levels up to 50 mg/kg (300 mg/m2) in male rats and 75 mg/kg (450 mg/m2) in female rats. These dosages are 1.6 and 2.4 times the maximum daily human dosage of 185 mg/m2, respectively.
Pregnancy
Tramadol has been shown to be embryotoxic and fetotoxic in mice, (120 mg/kg or 360 mg/m2), rats (≥25 mg/kg or 150 mg/m2) and rabbits (≥75mg/kg or 900 mg/m2) at maternally toxic dosages, but was not teratogenic at these dose levels. These dosages on an mg/m2 basis are 1.9, 0.8 and 4.9 times the maximum daily human dosage (185 mg/m2) for mouse, rat and rabbit, respectively.
No drug related teratogenic effects were observed in progeny of mice (up to 140 mg/kg or 420 mg/m2), rats (up to 80 mg/kg or 480 mg/m2) or rabbits (up to 300 mg/kg or 3600 mg/m2) treated with tramadol by various routes. Embryo and fetal toxicity consisted primarily of decreased fetal weights, skeletal ossification and increased supernumerary ribs in maternally toxic dose levels. Transient delays in developmental or behavioral parameters were also seen in pups from rat dams allowed to deliver. Embryo and fetal lethality were reported only in one rabbit study at 300 mg/kg (3600 mg/m2), a dose that would cause extreme maternal toxicity in the rabbit. The dosages listed for mouse, rat and rabbit are 2.2, 2.6 and 19.4 times the maximum daily human dosage (185 mg/m2), respectively.
Tramadol was evaluated in peri- and post-natal studies in rats. Progeny of dams receiving oral (gavage) dose levels of 50 mg/kg (300 mg/m2 or 1.6 times the maximum daily human Tramadol Hydrochloride Extended-Release Tablets dosage) or greater had decreased weights, and pup survival was decreased early in lactation at 80 mg/kg (480 mg/m2 or 2.6 times the maximum daily human dose).
There are no adequate and well-controlled studies in pregnant women. Tramadol Hydrochloride Extended-Release Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Neonatal seizures, neonatal withdrawal syndrome, fetal death and stillbirth have been reported during post-marketing surveillance of tramadol immediate-release products.
Tramadol Hydrochloride Extended-Release Tablets should not be used in pregnant women prior to or during labor unless the potential benefits outweigh the risks. Safe use in pregnancy has not been established. Chronic use during pregnancy may lead to physical dependence and post-partum withdrawal symptoms in the newborn (see DRUG ABUSE AND ADDICTION). Tramadol has been shown to cross the placenta. The mean ratio of serum tramadol in the umbilical veins compared to maternal veins was 0.83 for 40 women given tramadol during labor.
The effect Tramadol Hydrochloride Extended-Release Tablets, if any, on the later growth, development and functional maturation of the child is unknown.
Tramadol Hydrochloride Extended-Release Tablets are not recommended for obstetrical preoperative medication or for post-delivery analgesia in nursing mothers because its safety in infants and newborns has not been studied. Following a single IV 100 mg dose of tramadol, the cumulative excretion in breast milk within 16 hours postdose was 100 µg of tramadol (0.1% of the maternal dose) and 27 µg of M1.
The safety and efficacy of Tramadol Hydrochloride Extended-Release Tablets in patients under 16 years of age has not been established. The use of Tramadol Hydrochloride Extended-Release Tablets in the pediatric population is not recommended.
In general, caution should be used when selecting the dose for an elderly patient. Usually, dose administration should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
In 12-week clinical trials, Tramadol Hydrochloride Extended-Release Tablets were administered to 534 patients aged 65 years and older. Of those, 68 patients were 75 years of age and older. Comparable incidence rates of patients experiencing adverse events were observed for patients older than 65 years of age compared with younger patients (< 65 years of age), except constipation for which the incidence was higher in older patients. Tramadol Hydrochloride Extended-Release Tablets should be used with caution in patients older than 75 years of age (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
TRAMADOL HYDROCHLORIDE ADVERSE REACTIONS
Tramadol Hydrochloride Extended-Release Tablets were administered to a total of 2707 subjects (2406 patients and 301 healthy volunteers) during clinical studies, including four randomized double-blind studies (treatment ≥ 12 weeks) and two open-label long-term studies (treatment up to 12 months) in patients with moderate to severe pain due to osteoarthritis of the knee. A total of 844 patients were exposed to Tramadol Hydrochloride Extended-Release Tablets for 12 weeks, 493 patients for 6 months and 243 patients for 12 months. Treatment emergent adverse events increased with dose from 100 mg to 300 mg in the three twelve-week, randomized, double-blind, placebo-controlled studies (Table 2).
| *Due to the difference in study design of MDT3-005, only the results of the double-blind phase of the study are presented and the dose specific results include maintenance period data only. | |||||
| ADVERSE EVENTS (MEDRA Preferred Terms) |
Tramadol Hydrochloride
Extended-Release Tablets |
Placebo | |||
| 100 mg | 200 mg | 300 mg | Total* | ||
|
N=216
|
N=311
|
N=530
|
N=1095
|
N=668
|
|
| Nausea | 28 (13%) | 42 (14%) | 76 (14%) | 179 (16%) | 37 (6%) |
| Constipation | 21 (10%) | 36 (12%) | 52 (10%) | 140 (13%) | 26 (4%) |
| Dizziness | 16 (7%) | 28 (9%) | 52 (10%) | 106 (10%) | 18 (3%) |
| Somnolence | 11 (5%) | 22 (7%) | 23 (4%) | 77 (7%) | 12 (2%) |
| Vomiting | 7 (3%) | 16 (5%) | 31 (6%) | 58 (5%) | 4 (1%) |
| Pruritus | 9 (4%) | 15 (5%) | 18 (3%) | 51 (5%) | 7 (1%) |
| Headache | 10 (5%) | 9 (3%) | 15 (3%) | 41 (4%) | 21 (3%) |
| Sweating increased | 1 (0%) | 9 (3%) | 14 (3%) | 35 (3%) | 5 (1%) |
| Dry mouth | 7 (3%) | 13 (4%) | 6 (1%) | 32 (3%) | 8 (1%) |
| Fatigue | 6 (3%) | 7 (2%) | 9 (2%) | 26 (2%) | 6 (1%) |
| Anorexia | 4 (2%) | 4 (1%) | 10 (2%) | 25 (2%) | 2 (0%) |
| Vertigo | 2 (1%) | 3 (1%) | 6 (1%) | 21 (2%) | 3 (0%) |
| Insomnia | 2 (1%) | 6 (2%) | 9 (2%) | 18 (2%) | 8 (1%) |
The majority of patients who experienced the most common adverse events (≥5%) reported mild to moderate symptoms. Less than 3% of adverse events were rated as severe. Overall, onset of these adverse events usually occurred within the first two weeks of treatment.
Ear and labyrinth disorders: vertigo
Gastrointestinal disorders: abdominal pain, diarrhea, dry mouth, dyspepsia, upper abdominal pain
General disorders: fatigue, weakness
Investigations: weight decreased
Metabolism and nutrition disorders: anorexia
Musculoskeletal and connective tissue disorders: arthralgia
Nervous system disorders: headache, tremor
Psychiatric disorders: anxiety, insomnia
Skin and subcutaneous tissue disorders: pruritus, sweating increased
Vascular disorders: hot flushes
Blood and lymphatic system disorders: anemia, thrombocytopenia
Cardiac disorders: bradycardia
Eye disorders: blurred vision, visual disturbance
Gastrointestinal disorders: abdominal discomfort, abdominal distension, abdominal tenderness, change in bowel habit, constipation aggravated, diverticulitis, diverticulum, dyspepsia aggravated, dysphagia, fecal impaction, gastric irritation, gastritis, gastrointestinal hemorrhage, gastrointestinal irritation, gastro-esophageal reflux disease, lower abdominal pain, pancreatitis aggravated, rectal hemorrhage, rectal prolapse, retching
General disorders: asthenia, malaise
Hepatobiliary disorders: biliary tract disorder, cholelithiasis
Immune system disorders: hypersensitivity
Investigations: alanine aminotransferase decreased, alanine aminotransferase increased, aspartate aminotransferase decreased, aspartate aminotransferase increased, blood amylase increased, blood creatinine increased, blood in stool, blood potassium abnormal, blood pressure increased gamma glutamyltransferase increased
Metabolism and nutrition disorders: appetite decreased, dehydration
Nervous system disorders: ataxia, disturbance in attention, dysarthria, gait abnormal, headache aggravated, mental impairment, sedation, seizure, sleep apnea syndrome, syncope, tremor
Psychiatric disorders: abnormal behavior, agitation, anxiety, confusion, depression, emotional disturbance, euphoric mood, indifference, irritability, libido decreased, nervousness, sleep disorder
Renal and urinary disorders: difficulty in micturition, urinary hesitation, urinary retention
Reproductive system and breast disorders: erectile dysfunction, sexual dysfunction
Respiratory, thoracic and mediastinal disorders: dyspnea
Skin and subcutaneous tissue disorders: allergic dermatitis, cold sweat, dermatitis, night sweats, pallor, generalized pruritus, urticaria
Vascular disorders: flushing, hypertension, hypotension, orthostatic hypotension
OVERDOSAGE
Acute overdosage with tramadol can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, hypotension and death.
Death due to overdose has been reported with abuse and misuse of tramadol, by ingesting, inhaling, or injecting the crushed tablets. The risk of fatal overdose is further increased when tramadol is abused concurrently with alcohol and other CNS depressants, including other opioids.
In the treatment of tramadol overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
While naloxone will reverse some (but not all) symptoms caused by overdosage with tramadol, the risk of seizures is also increased with naloxone administration. In animals, convulsions following the administration of toxic doses of tramadol could be suppressed with barbiturates or benzodiazepines but were increased with naloxone. Naloxone administration did not change the lethality of an overdose in mice. Hemodialysis is not expected to be helpful in an overdose because it removes less than 7% of the administered dose in a 4-hour dialysis period.
TRAMADOL HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Tramadol Hydrochloride Extended-Release Tablets should be taken once a day. The tablets should be swallowed whole with liquid and not split, chewed, dissolved or crushed. Tramadol Hydrochloride Extended-Release Tablets produce a continuous release of active ingredient over 24 hours: a repeat dosage within 24 hours is not recommended.
Patients Not Currently on Tramadol Immediate-Release Products:
Treatment with Tramadol Hydrochloride Extended-Release Tablets should be initiated at a dose of 100 mg/day. Daily doses should be titrated by 100 mg/day increments every 2 to 3 days (i.e., start 200 mg/day on day 3 or 4 of therapy) to achieve a balance between adequate pain control and tolerability for the individual patient. For patients requiring the 300 mg daily dose, titration should take at least 4 days (i.e. 300 mg/day on day 5). The usual daily dose is 200 or 300 mg. The daily dose and titration should be individualized for each patient. Therapy should be continued with the lowest effective dose. Tramadol Hydrochloride Extended-Release Tablets should not be administered at a dose exceeding 300 mg per day.
Clinical experience suggests that signs and symptoms of withdrawal may be reduced by tapering medication when discontinuing tramadol therapy.
Patients Currently on Tramadol Immediate-Release Products:
For patients maintained on tramadol immediate release (IR) products, the 24-hour tramadol IR dose should be calculated and the patient should be initiated on a total daily dose of Tramadol Hydrochloride Extended-Release Tablets rounded down to the next lowest 100 mg increment. The dose may subsequently be individualized according to patient need. Due to limitations in flexibility of dose selection with Tramadol Hydrochloride Extended-Release Tablets, some patients maintained on tramadol IR products may not be able to convert to Tramadol Hydrochloride Extended-Release Tablets. Tramadol Hydrochloride Extended-Release Tablets should not be administered at a dose exceeding 300 mg per day. Do not use Tramadol Hydrochloride Extended-Release Tablets with other tramadol products (see WARNINGS).
Good pain management practice dictates that analgesic dose be individualized according to patient need using the lowest beneficial dose. Studies with tramadol products in adults have shown that starting at the lowest possible dose and titrating upward will result in fewer discontinuations and increased tolerability.
Tramadol Hydrochloride Extended-Release Tablets should not be used in patients with:
- Creatinine clearance less than 30 mL/min,
- Hepatic impairment
(see WARNINGS, Use in Renal and Hepatic Disease).
In general, dose selection for patients over 65 years of age who may have decreased hepatic or renal function, or other concomitant diseases, should be initiated cautiously, usually starting at the low end of the dosing range. Tramadol Hydrochloride Extended-Release Tablets should be administered with greater caution at the lowest effective dose in patients over 75 years, due to the potential for greater frequency of adverse events in this population.
HOW SUPPLIED
Tramadol Hydrochloride Extended-Release Tablets are supplied in a number of packages and dose strengths:
100-mg, white, beveled edge, round biconvex tablets, plain on one side and printed "PP 100" in black ink on the other side.
Bottle of 30 tablets – NDC 42858-901-03
200-mg, white, beveled edge, round biconvex tablets, plain on one side and printed "PP 200" in black ink on the other side.
Bottle of 30 tablets – NDC 42858-902-03
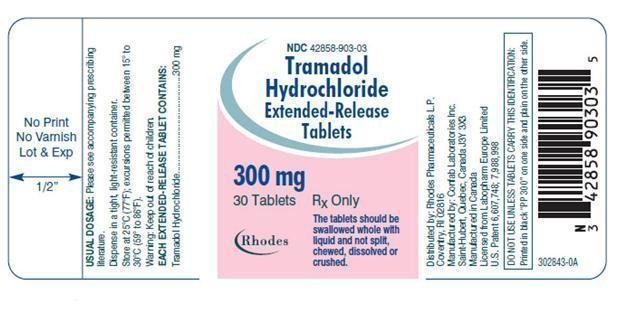
300-mg, white, beveled edge, round biconvex tablets, plain on one side and printed "PP 300" in black ink on the other side.
Bottle of 30 tablets – NDC 42858-903-03
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
100 mg Bottles of 30 tablets
Rx Only NDC 42858-901-03

200 mg Bottles of 30 tablets
Rx Only NDC 42858-902-03

300 mg Bottles of 30 tablets
Rx Only NDC 42858-903-03
Tramadol HydrochlorideTramadol Hydrochloride TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tramadol HydrochlorideTramadol Hydrochloride TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tramadol HydrochlorideTramadol Hydrochloride TABLET, EXTENDED RELEASE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||