Timewise Day Solution sunscreen SPF 35
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients ; HOMOSALATE (10% w/w) ,OCTISALATE(5% w/w),OXYBENZONE(4%w/w), OCOCRYLENE (2% w/w), AVOBENZONE(2% w/w)
Warnings; For external use only. Not to be swalloed. Avoid contact with the eyes. Discontinue use if signs of irritation or rash appear.
Keep out of reach of children. Do not stay too long in the sun, even while using a sunscreen product.
Uses
Indication; Broad-spectrum UVA/UVB protection.
Water,Dimethicone, Butylene Glycol,Styrene/Acrylates Copolymer,C12-15 Alkyl Benzoate,Dicapryl Carbonate,Glycerin,Ceteareth-25,Dimethicone Crosspolymer,Magnesium Aluminum Silicate, Acetyl Dipeptide-1,Cetyl Ester, Plankton Extract,Hydrolyzed Algin, Adenosin, Pentylene Glycol , Tocopheryl Acetate,Crithmum Marithimum Extract,Ascorbic Acid,Hydrogenated Lecithin,Hydroxyethylcellulose,Dipropylene Glycol,Dibenzoate Acrylate / C12-22 Alkyl Methacrylate Crosspolymer ,Disodium Ethylene Dicocamide PEG-15 Disulfate,Sorbic Acid ,Caprylyl Glycol, PPG-15 Stearyl Ether Benzoate,Propylene Glycol , Xanthan Gum, Cyclopentasiloxane, PEG-75,PEG-150,PEG-6 Cetyl Dimethicone,PEG-4 Laurate,Laureth-3 ,Disodium EDTA , Ammonium Hydroxide,Iodopropyl Butylcarbamate,Methylparaben,Phenoxyethanol
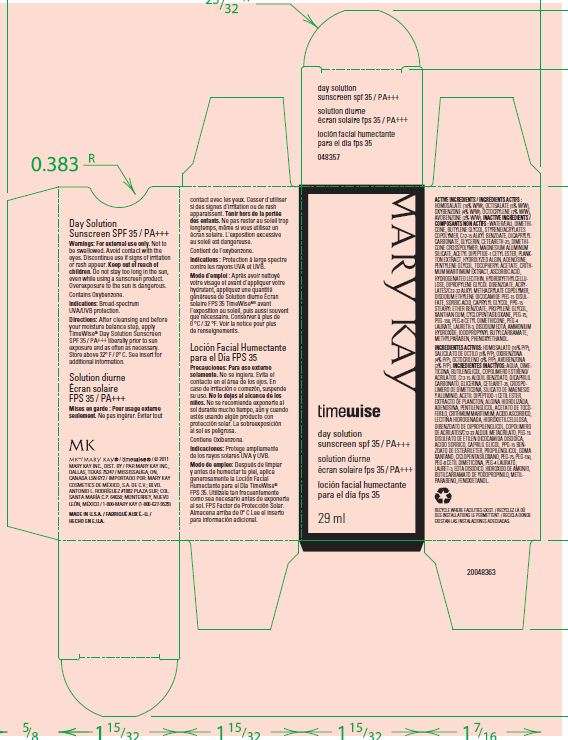
Timewise Day Solution sunscreen SPF 35Homosalate,Octisalate,Oxybenzone,Octocrylene,Avobenzone CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||