Diamond Animal Health, Inc..
Heska Corporation
ThyroMed Chewable Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
CAUTION:DESCRIPTION: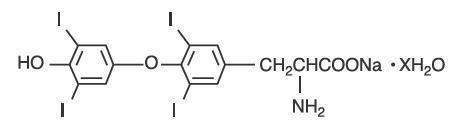
Levothyroxine Sodium Action
Levothyroxine sodium acts, as does endogenous thyroxine, to stimulate metabolism, growth, development and differentiation of tissues. It increases the rate of energy exchange and increases the maturation rate of the epiphyses. Levothyroxine sodium is absorbed rapidly from the gastrointestinal tract after oral administration. Following absorption, the compound becomes bound to the serum alpha globulin fraction. For purposes of comparison, 0.1 mg of levothyroxine sodium elicits a clinical response approximately equal to that produced by one grain (65 mg) of desiccated thyroid.
Uses
INDICATIONS:
43ThyroMed43
HYPOTHYROIDISM IN THE DOG:
Hypothyroidism usually occurs in middle-aged and older dogs although the condition will sometimes be seen in younger dogs of the larger breeds. Neutered animals of either sex are also frequently affected, regardless of age. The following are clinical signs of hypothyroidism in dogs:
Dermatological
Metabolic
Musculoskeletal
Reproductive
Gastrointestinal
Clinical pathological
CONTRAINDICATIONS:
Levothyroxine sodium therapy is contraindicated in thyrotoxicosis, acute myocardial infarction and uncorrected adrenal insufficiency. Use in pregnant bitches has not been evaluated.
PRECAUTIONS:
KEEP OUT OF REACH OF CHILDREN
ADVERSE REACTIONS:
DOSAGE:
ADMINISTRATION:
HOW SUPPLIED:
ThyroMed® Chewable Tablets are available in 0.1 mg, 0.2 mg, 0.3 mg, 0.4 mg, 0.5 mg, 0.6 mg, 0.7
mg and 0.8 mg strength chewable tablets. Tablets are bilaterally scored. The tablets are packaged in
bottles of 180 tablets (with childproof caps) and 1,000 tablets.
STORAGE:
Store at controlled room temperature; 15° C to 30° C (59° F to 86° F) and protect from light.
Dispense product in this container or another light, light-resistant container as defined in the USP.
REFERENCES:
Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction. W.B. Saunders, Co.,
Philadelphia, PA 1996: 68−117.
Scott DW, Miller WH, Griffen CE. Small Animal Dermatology. W.B. Saunders, Co., Philadelphia,
PA 1995: 237−8, 894−5.
Nelson RW. Current Veterinary Therapy X. R.W. Kirk (ed), W.B. Saunders, Co., Philadelphia, PA 1989:
993−997.
Evinger JV, Nelson RW. J Am Vet Med Assoc 1984; 185(3): 314−316.
Manufactured by Diamond Animal Health, Inc.
A wholly owned subsidiary of Heska Corporation
Des Moines, Iowa 50327
HESKA
Smarter, TogetherTM
ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-201-11
0.1mg
(100 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-202-11
0.2mg
(200 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-203-11
0.3mg
(300 mcg)
180 Tablets


ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-205-11
0.5mg
(500 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-206-11
0.6mg
(600 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-207-11
0.7mg
(700 mcg)
180 Tablets

ThyroMed®
Chewable Tablets
(Levothyroxine Sodium)
N 053701-281-11
0.8 mg
(800 mcg)
180 Tablets

ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-201 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.1 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;1 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-201-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-201-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|
ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-202 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.2 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;2 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-202-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-202-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|
ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-203 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.3 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;3 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-203-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-203-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|
ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-204 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.4 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;4 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-204-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-204-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|
ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-205 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.5 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;5 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-205-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-205-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|
ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-206 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.6 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;6 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-206-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-206-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|
ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-207 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.7 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;7 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-207-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-207-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|
ThyroMed
levothyroxine sodium TABLET, CHEWABLE
Product Information
|
|
Product Type
|
Prescription animal drug label |
Item Code (Source)
|
NDC:53701-208 |
|
Route of Administration
|
ORAL |
DEA Schedule
|
|
Active Ingredient/Active Moiety
|
|
Ingredient Name
|
Basis of Strength
|
Strength
|
|
LEVOTHYROXINE SODIUM LEVOTHYROXINE |
|
0.8 mg
|
Product Characteristics
|
|
Color
|
Size
|
Imprint Code
|
Shape
|
|
brown |
10 mm |
0;8 |
OVAL |
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:53701-208-11 |
180 in 1 BOTTLE, PLASTIC |
|
|
|
2 |
NDC:53701-208-12 |
1000 in 1 BOTTLE, PLASTIC |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2009-06-01 |
|
|








