Theophylline
FULL PRESCRIBING INFORMATION: CONTENTS*
- THEOPHYLLINE DESCRIPTION
- PHARMACOKINETICS
- INDICATIONS & USAGE
- THEOPHYLLINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- CLINICAL PHARMACOLOGY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- THEOPHYLLINE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
THEOPHYLLINE DESCRIPTION
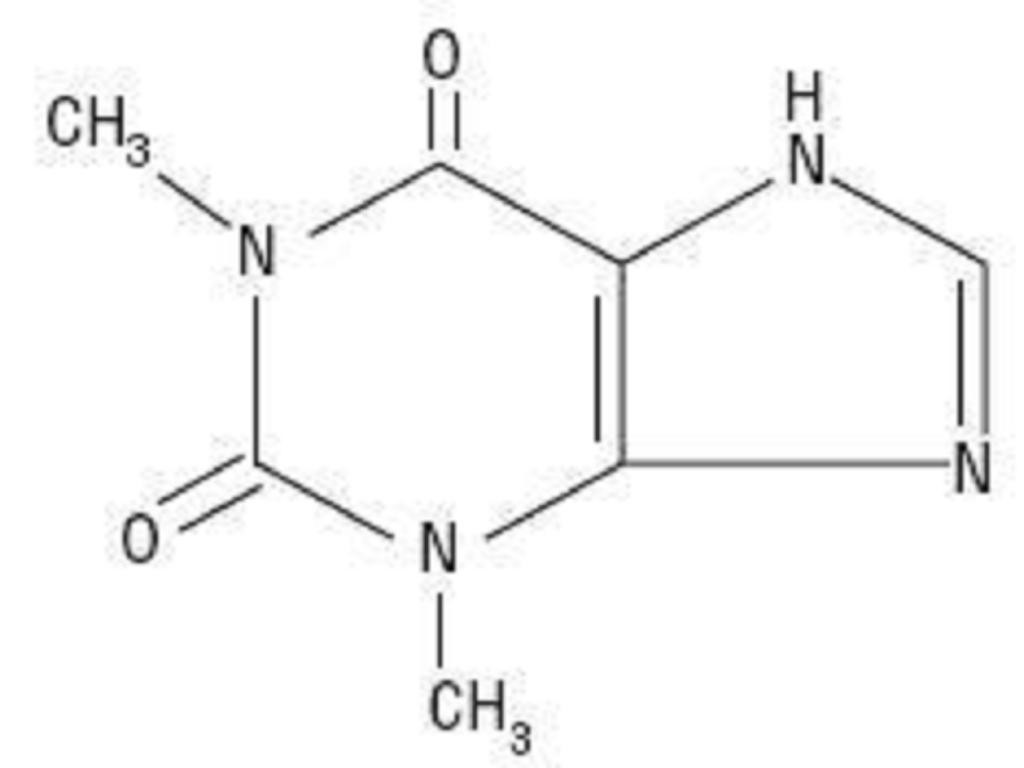
DOSAGE AND ADMINISTRATION
PHARMACOKINETICS
OverviewTable ITable IIPRECAUTIONS, Laboratory Tests
Table I. Mean and range of total body clearance and half-life of theophylline related to age and altered physiological states.*
PopulationTotal body clearanceHalf-life mean (range)Characteristicsmean (range)Age(mL/kg/min)(hr)Premature neonatespostnatal age 3 - 15 days 0.29 (0.09 to 0.49)30 (17 to 43)postnatal age 25 - 27days 0.64 (0.04 to 1.2)20 (9.4 to 30.6)Term infantspostnatal age 1 - 2 daysNR25.7 (25 to 26.5)postnatal age 3 - 30weeksNR11 (6 to 29)Children1 to 4 years1.7 (0.5 to 2.9)3.4 (1.2 to 5.6)4 to 12 years1.6 (0.8 to 2.4)NR13 to 5 years0.9 (0.48 to 1.3)NR6 to 17 years1.4 (0.2 to 2.6)3.7 (1.5 to 5.9)Adults (16 to 60 years)otherwise healthynonsmoking asthmatics0.65 (0.27 to 1.03)8.7 (6.1 to 12.8)Elderly (>60 years)nonsmokers with normalcardiac, liver and renalfunction0.41 (0.21 to 0.61)9.8 (1.6 to 18)Concurrent illness or altered physiological stateAcute pulmonary edema0.33(0.07 to 2.45)19(3.1 to 82)COPD->60 years, stablenonsmoker > 1 year0.54 (0.44 to 0.64)11 (9.4 to 12.6)COPD with corpulmonale0.48 (0.08 to 0.88)NRCystic fibrosis(14 to 28 years)1.25 (0.31 to 2.2)6.0 (1.8 to 10.2)Fever associated with acuteviral respiratory illness(children 9 - 15 years)NR.0 (1.0 to 13)Liver disease - cirrhosis0.31(0.1 to 0.7)32(10 to 56)acute hepatitis0.35 (0.25 to 0.45)19.2 (16.6 to 21.8)cholestasis0.65 (0.25 to 1.45)14.4 (5.7 to 31.8)Pregnancy - 1st trimesterNR8.5 (3.1 to 13.9)2nd trimesterNR8.8 (3.8 to 13.8)3rd trimesterNR13.0 (8.4 to 17.6)Sepsis with multi-organ18.8 (6.failure0.47 (0.19 to 1.9)18.8 (6.3 to 21.4)Thyroid disease-hypothyroid0.38 (0.13 to 0.57)11.6 (8.2 to 25)hyperthyroid0.8 (0.68 to 0.97)4.5 (3.7 to 5.6)*For various North American patient populations from literature reports. Different rates of elimination and consequent dosage requirements have been observed among other peoples.
Reported range or estimated range (mean +/- 2 SD) where actual range not reported.
NR = not reported or not reported in a comparable format.
Median.
NOTE: In addition to the factors listed above, theophylline clearance is increased and half-life
decreased by low carbohydrate/high protein diets, parenteral nutrition, and daily consumption
of charcoal-broiled beef. A high carbohydrate/low protein diet can decrease the clearance and
prolong the half-life of theophylline.
Absorption
Single-Dose Study
Multiple-Dose Study
Once-a-Day Dosing
Distribution
Metabolism
DOSAGE AND ADMINISTRATIONTable VI
Excretion
WARNINGS
Serum Concentrations at Steady-State
Special Populations (see Table I for mean clearance and half-life values)
Geriatric
WARNINGS
Pediatrics
WARNINGSWARNINGSDOSAGE AND ADMINISTRATION
Gender
Race
Renal Insufficiency
WARNINGS
Hepatic Insufficiency
WARNINGS
Congestive Heart Failure (CHF)
WARNINGS
Smokers
WARNINGS
Fever
WARNINGS
Miscellaneous
WARNINGS
Clinical Studies
INDICATIONS & USAGE
THEOPHYLLINE CONTRAINDICATIONS
WARNINGS
Concurrent IllnessCardiac arrhythmias (not including bradyarrhythmias).
Age
Concurrent Diseases
Cessation of Smoking
Drug Interactions
PRECAUTIONS, Drug Interactions, Table II
DOSAGE AND ADMINISTRATIONDosing Guidelines,Table VI
Dosage Increases
PRECAUTIONS, Laboratory Tests
DOSAGE AND ADMINISTRATIONTable VI
PRECAUTIONS
GeneralWARNINGSDOSAGE AND ADMINISTRATIONTable V
Monitoring Serum Theophylline Concentrations
Effects on Laboratory Tests
INFORMATION FOR PATIENTS
DRUG INTERACTIONS
Drug-Drug InteractionsTable II. Clinically significant drug interactions with theophylline.*
DrugType of InteractionEffect*
Table III. Drugs that have been documented not to interact with theophylline or drugs that produce no clinically significant interaction with theophylline.*
*
Drug-Food Interactions
The Effect of Other Drugs on Theophylline Serum Concentration Measurements
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Category CCLINICAL PHARMACOLOGY
Mechanism of ActionSerum Concentration-Effect Relationship
NURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGYTable IWARNINGSDOSAGE AND ADMINISTRATIONTable VGERIATRIC USE
DOSAGE AND ADMINISTRATIONTHEOPHYLLINE ADVERSE REACTIONS
OVERDOSAGEDOSAGE AND ADMINISTRATIONTable VTable IV. Manifestations of theophylline toxicity.*Percentage of patients reported with sign or symptom
Acute OverdoseChronic Overdosage(Large Single(Multiple ExcessiveIngestion)Doses)Sign/SymptomStudy 1 Study 2Study 1 Study 2Asymotomatic(n=157)(n=14)(n=92) (n=102)GastointestinalNR0NR6Vomiting73 9330 61Abdominal PainNR21NR12DiarrheaNR0NR14HematemesisNR0NR2Metabolic/OtherHypokalemia85 7944 43Hyperglycemia98 NR18 NRAcid/base disturbance34 219 5RhabdomyolysisNR7NR0CardiovascularSinus tachycardia100 86100 62Other supraventricular2 2112 14tachycardiasVentricular premature beats3 2110 19Atrial fibrillation or flutter1 NR12 NRMultifocal atrial tachycardia0 NR2 NRVentricular arrhythmias7 1440 0hemodynamic instabilityHypotension/shockNR21NR8NeurologicNervousnessNR64NR21Tremors38 2916 14DisorientationNR7NR11Seizures5 1414 5Death3 2110 4
*
NR = Not reported in a comparable manner.
OVERDOSAGE
GeneralTable IV
Overdose Management
OVERDOSAGEExtracorporeal Removal
Specific Recommendations
Acute Overdose
OVERDOSAGEExtracorporeal Removal
OVERDOSAGEExtracorporeal Removal
OVERDOSAGEExtracorporeal Removal
OVERDOSAGEExtracorporeal Removal
Extracorporeal Removal
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGYDrug InteractionsDrug-Food InteractionsGeneral Considerations
Table VPRECAUTIONSLaboratory TestsDOSAGE AND ADMINISTRATIONTable VIWARNINGS
WARNINGSPRECAUTIONS
Table VTable VI
Table V. Dosing initiation and titration (as anhydrous theophylline).*
A.Children (6 to 15 years) and adults (16 to 60 years)without risk factors for impaired clearance.******B.Patients With Risk Factors For Impaired Clearance, The Elderly (>60 Years), And Those In Whom It Is Not Feasible To Monitor Serum Theophylline Concentrations:WARNINGSWARNINGS*
Table VI. Dosage adjustment guided by serum theophylline concentration.
Peak SerumDosage AdjustmentConcentration***WARNINGS
Once-Daily Dosing
HOW SUPPLIED
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


TheophyllineTheophylline TABLET, EXTENDED RELEASE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!