Tetracycline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TETRACYCLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TETRACYCLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- TETRACYCLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- ANIMAL PHARMACOLOGY & OR TOXICOLOGY
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
TETRACYCLINE HYDROCHLORIDE DESCRIPTION
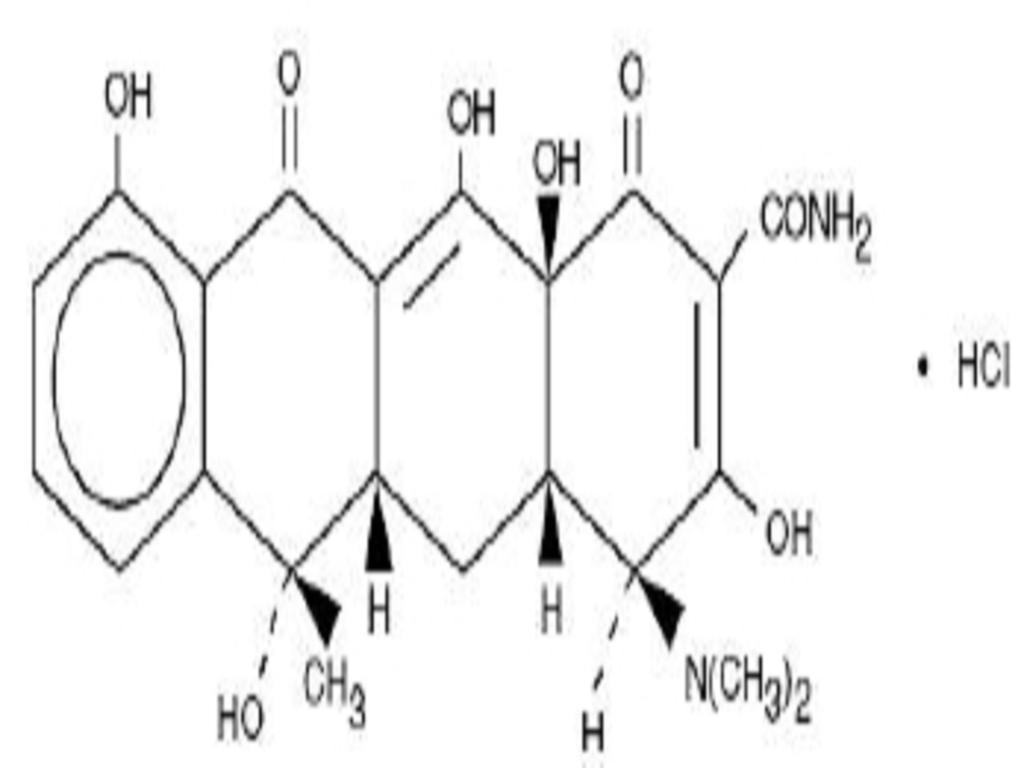
CLINICAL PHARMACOLOGY
Microbiology
Gram-negative Bacteria
Gram-positive Bacteria
Other microorganisms
Susceptibility Testing
INDICATIONS & USAGE
-
● Upper respiratory tract infections caused by Streptococcus pyogenes, Streptococcus pneumoniae and Hemophilus influenzae. Note: Tetracycline should not be used for streptococcal disease unless the organism has been demonstrated to be susceptible.
-
● Lower respiratory tract infections caused by Streptococcus pyogenes, Streptococcus pneumoniae, Mycoplasma pneumoniae (Eaton agent, and Klebsiella sp.)
-
● Skin and soft tissue infections caused by Streptococcus pyogenes, Staphylococcus aureaus. (Tetracyclines are not the drugs of choice in the treatment of any type of staphylococcal infections.)
-
● Infections caused by rickettsia including Rocky Mountain spotted fever, typhus group infections, Q fever, rickettsialpox.
-
● Psittacosis or ornithosis caused by Chlamydia Psittaci.
-
● Infections caused by Chlamydia trachomatis such as uncomplicated urethral, endocervical or rectal infections, inclusion conjunctivitis, trachoma, and lymphogranuloma venereum.
-
● Granuloma inquinale caused by Calymmatobacterium granulomatis.
-
● Relapsing fever caused by Borrelia sp.
-
● Bartonellosis caused by Bartonella bacilliformis.
-
● Chancroid caused by Hemophilus ducreyi.
-
● Tularemia caused by Francisella tularensis.
-
● Plaque caused by Yersinia pestis.
-
● Cholera caused by Vibrio cholerae.
-
● Brucellosis caused by Brucella species (tetracycline may be used in conjunction with an aminoglycoside).
-
● Infections due to Campylobacter fetus.
-
● As adjunctive therapy in intestinal amebiasis caused by Entamoeba histolytica.
-
● Urinary tract infections caused by susceptible strains of Escherichia coli, Klebsiella, etc.
-
● Other infections caused by susceptible gram-negative organisms such as E. coli, Enterobacter aerogenes, Shigella sp., Acinetobacter sp., Klebsiella sp., and Bacteroides sp.
-
● In severe acne, adjunctive therapy with tetracycline may be useful.
-
● Syphilis and yaws caused by Treponema pallidum and pertenue, respectively,
-
● Vincent's infection caused by Fusobacterium fusiforme,
-
● Infections caused by Neisseria gonorrhoeae,
-
● Anthrax caused by Bacillus anthracis,
-
● Infections due to Listeria monocytogenes,
-
● Actinomycosis caused by Actinomyces species,
-
● Infections due to Clostridium species.
TETRACYCLINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects:Pregnancy Category D
Nonteratogenic Effects:
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
TETRACYCLINE HYDROCHLORIDE ADVERSE REACTIONS
Gastrointestinal:Teeth:
Skin:
Renal toxicity:
Liver:
Hypersensitivity reactions:
Blood:
Other:
OVERDOSAGE
DOSAGE & ADMINISTRATION
AdultsChildren above eight years of age
Concomitant therapy
HOW SUPPLIED
STORAGE AND HANDLING
ANIMAL PHARMACOLOGY & OR TOXICOLOGY
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Tetracycline HydrochlorideTetracycline Hydrochloride CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!