Terazosin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERAZOSIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
TERAZOSIN HYDROCHLORIDE DESCRIPTION
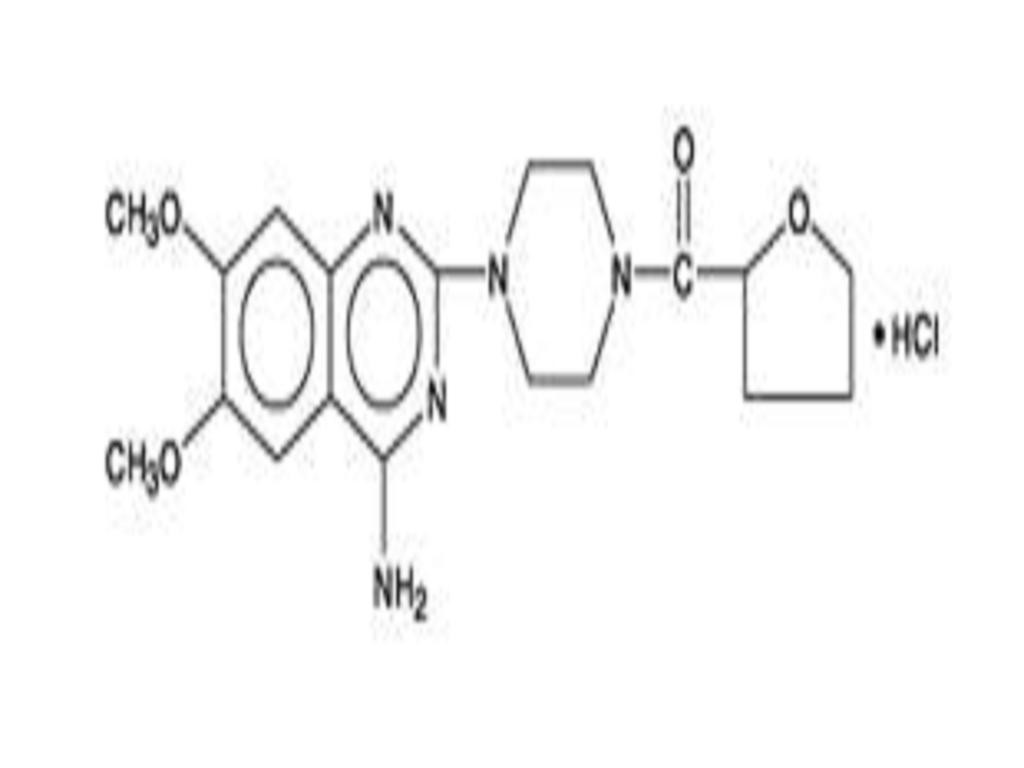
CLINICAL PHARMACOLOGY
Pharmacodynamics-
● p
-
● FIGURE 3. Mean Change in Peak Flow Rate from Baseline Long-Term, Open-Label, Non-Placebo Controlled Study (N=494)
Pharmacokinetics
INDICATIONS & USAGE
TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
GeneralINFORMATION FOR PATIENTS
DRUG INTERACTIONS
Use With Other Drugs
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
Benign Prostatic HyperplasiaHypertension
Post-marketing Experience
OVERDOSAGE
DOSAGE & ADMINISTRATION
Benign Prostatic Hyperplasia
Hypertension
HOW SUPPLIED
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
LABORATORY TESTS
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION


Terazosin HydrochlorideTerazosin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!