Terazosin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- TERAZOSIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- INDICATIONS & USAGE
- TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
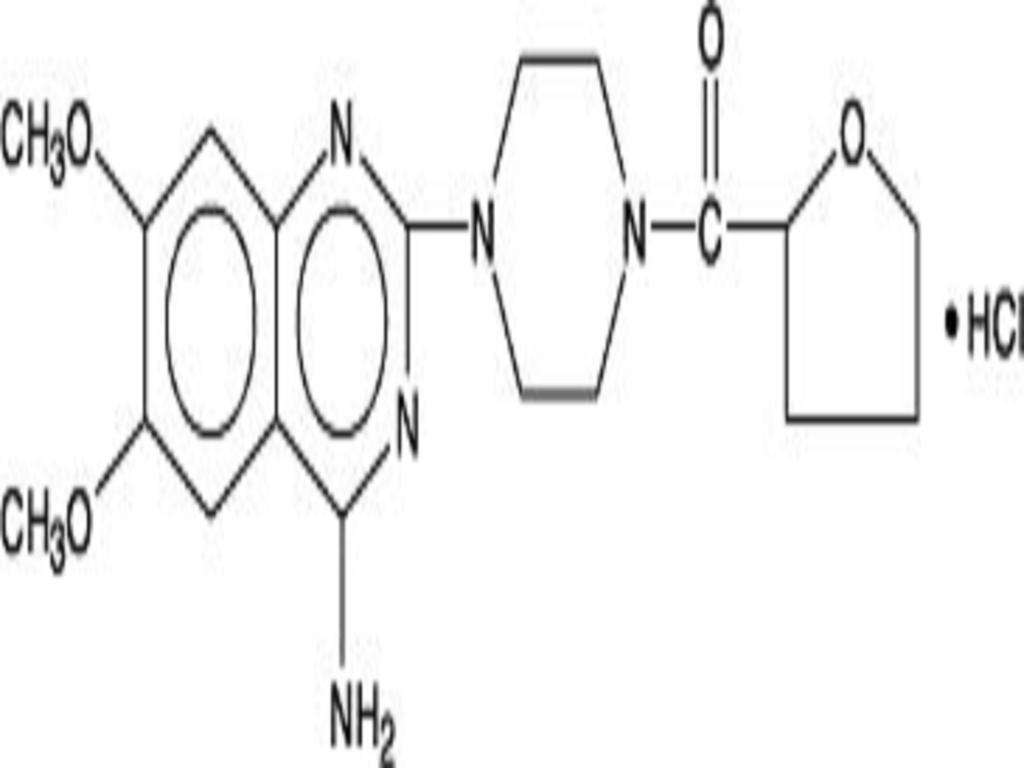
TERAZOSIN HYDROCHLORIDE DESCRIPTION
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
A. Benign Prostatic Hyperplasia (BPH)**
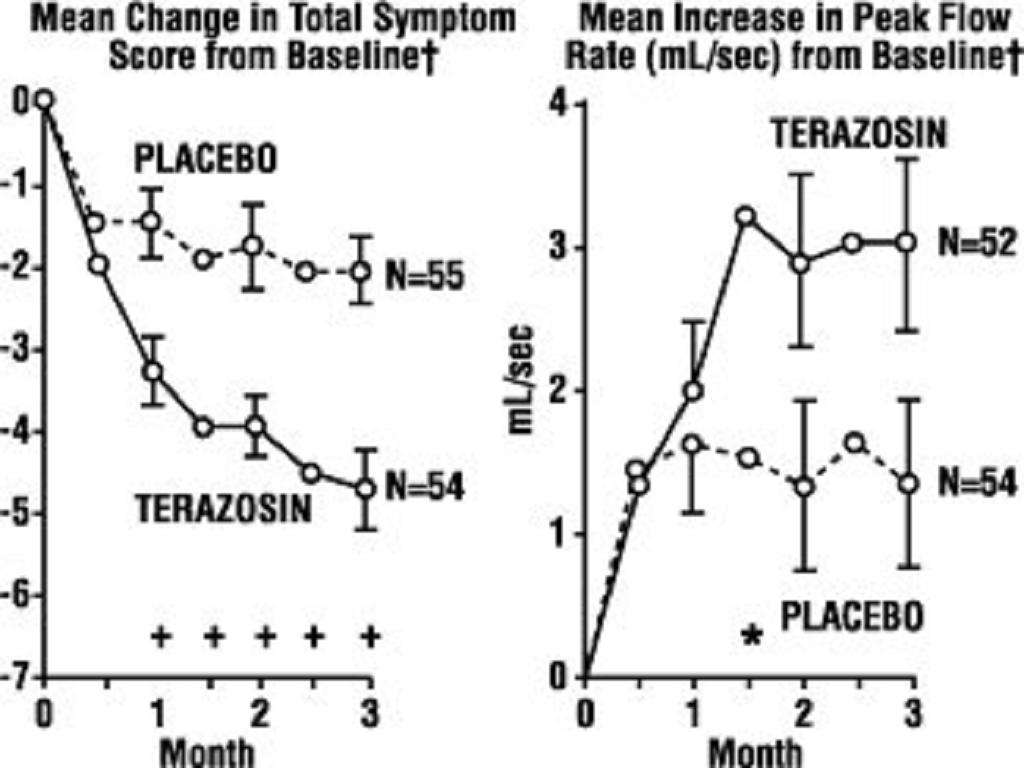
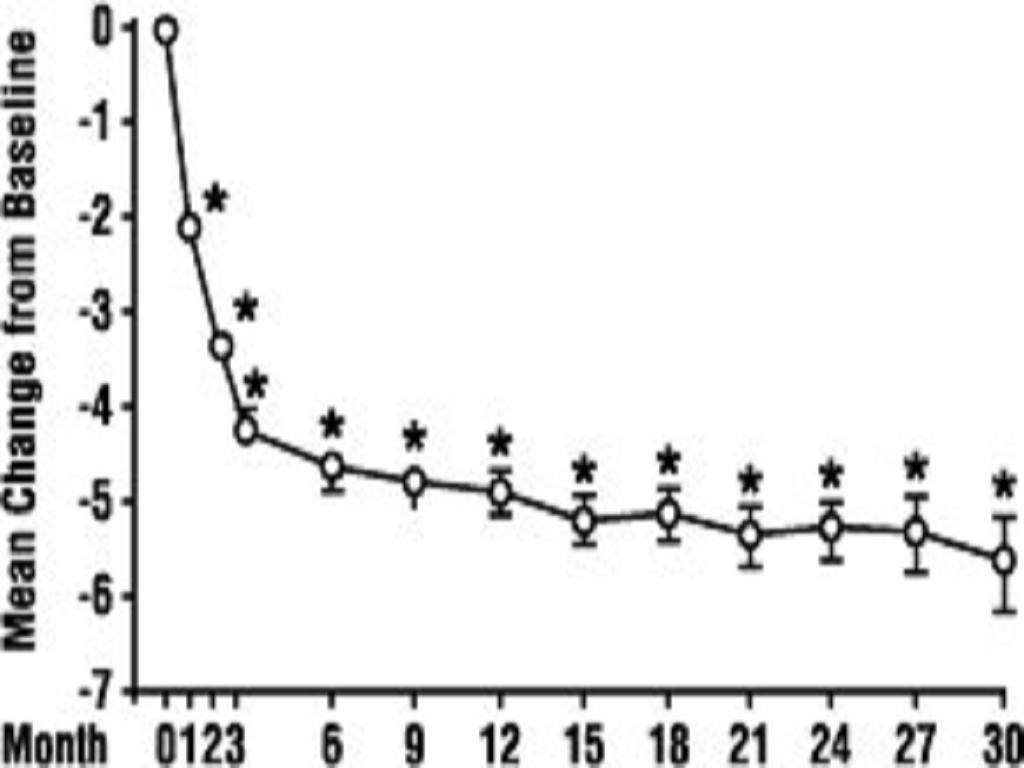
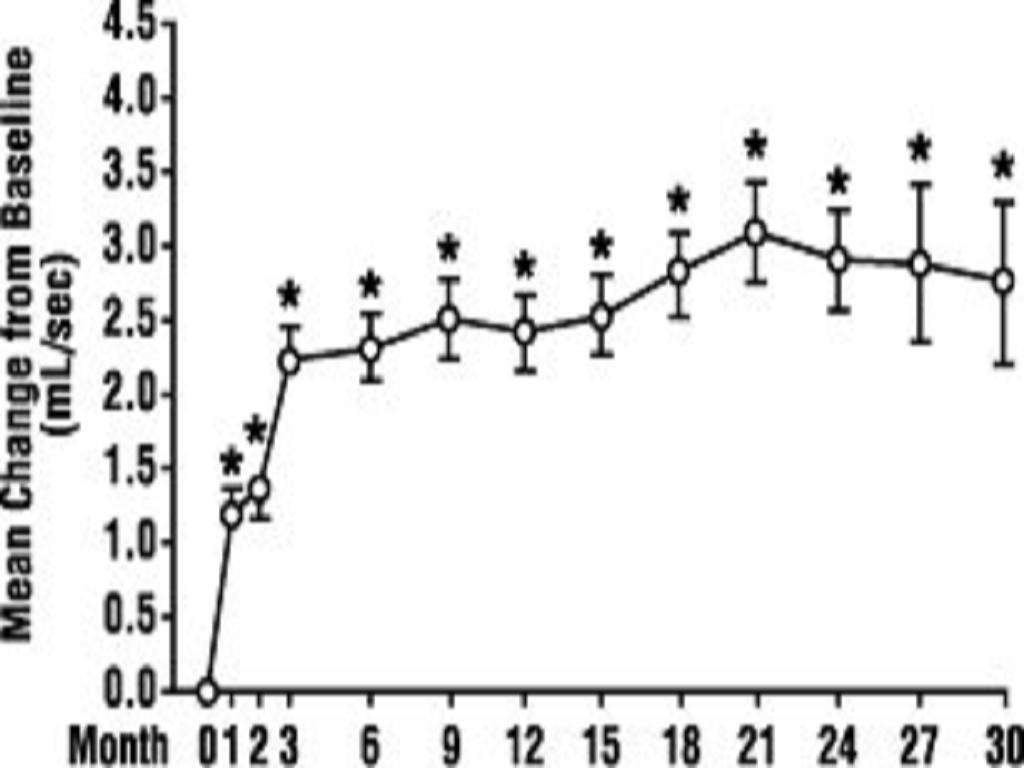
<*****
B. Hypertension
INDICATIONS & USAGE
TERAZOSIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Syncope andFirst-doseEffectCLINICAL PHARMACOLOGY
Priapism
PRECAUTIONS: Information for Patients
PRECAUTIONS
GeneralProstatic Cancer
Intraoperative Floppy Iris Syndrome (IFIS)
Orthostatic Hypotension
WARNINGS
INFORMATION FOR PATIENTS
PATIENT INFORMATIONLABORATORY TESTS
DRUG INTERACTIONS
Use With Other Drugs
DOSAGE AND ADMINISTRATION
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category C
Nonteratogenic Effects
NURSING MOTHERS
PEDIATRIC USE
TERAZOSIN HYDROCHLORIDE ADVERSE REACTIONS
Benign Prostatic HyperplasiaPRECAUTIONS
Body SystemTerazosinPlacebo(N = 636)(N = 360)BODY AS A WHOLE*CARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMMETABOLIC ANDNUTRITIONAL DISORDERSNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSESUROGENITAL SYSTEM*
Body SystemTerazosinPlacebo(N = 636)(N = 360)BODY AS A WHOLECARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSESUROGENITAL SYSTEM
Hypertension
Body SystemTerazosinPlacebo(N = 859)(N = 506)BODY AS A WHOLE*CARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMMETABOLIC ANDNUTRITIONAL DISORDERSMUSCULOSKETAL SYSTEMNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSESUROGENITAL SYSTEM*
Body as a Whole
Cardiovascular System
Digestive System
Metabolic/Nutritional Disorders
Musculoskeletal System
Nervous System
Respiratory System
Skin and Appendages
Special Senses
Urogenital System
Body SystemTerazosinPlacebo(N = 859)(N = 506)BODY AS A WHOLECARDIOVASCULAR SYSTEMDIGESTIVE SYSTEMMETABOLIC AND NUTRITIONALDISORDERSNERVOUS SYSTEMRESPIRATORY SYSTEMSPECIAL SENSES
Post-marketing Experience
PRECAUTIONS
DOSAGE & ADMINISTRATION
Benign Prostatic Hyperplasia
Initial Dose
Subsequent Doses
Use With Other Drugs
PRECAUTIONS
Hypertension
Initial Dose
Subsequent Doses
Use With Other Drugs
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATION-
● a weak or interrupted stream when urinating
-
● a feeling that you cannot empty your bladder completely
-
● a feeling of delay when you start to urinate
-
● a need to urinate often, especially at night, or
-
● a feeling that you must urinate right away.
-
● Program of monitoring orWatchful Waiting. Some men have an enlarged prostate gland, but no symptoms, or symptoms that are not bothersome. If this applies, you and your doctor may decide on a program of monitoring including regular checkups, instead of medication or surgery.
-
● Medication. There are different kinds of medication used to treat BPH. Your doctor has prescribed terazosin capsules for you. SeeWhat terazosin capsules do to treat BPHbelow.
-
● Surgery. Some patients may need surgery. Your doctor can describe several different surgical procedures to treat BPH. Which procedure is best depends on your symptoms and medical condition.
-
● Terazosin hydrochloride helps relieve the symptoms of BPH. It does NOT change the size of the prostate, which may continue to grow. However, a larger prostate does not necessarily cause more or worse symptoms.
-
● If terazosin capsules are helping you, you should notice an effect on your particular symptoms in 2 to 4 weeks of starting to take the medication.
-
● Even though you take terazosin capsules and they may help you, terazosin capsules may not prevent the need for surgery in the future.
-
● You should see an effect on your symptoms in 2 to 4 weeks. So, you will need to continue seeing your doctor to check your progress regarding your BPH and to monitor your blood pressure in addition to your other regular checkups.
-
● Your doctor has prescribed terazosin capsules for your BPH and not for prostate cancer. However, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). These checks should continue even if you are taking terazosin capsules. Terazosin capsules are not a treatment for prostate cancer.
-
● About Prostate Specific Antigen (PSA). Your doctor may have done a blood test called PSA. Your doctor is aware that terazosin capsules do not affect PSA levels. You may want to ask your doctor more about this if you have had a PSA test done.
-
● You will start with a 1 mg dose of terazosin capsules. The dose will be increased as your body gets used to the effect of the medication.
-
● Other side effects you could have while taking terazosin capsules include drowsiness, blurred or hazy vision, nausea, orpuffinessof the feet or hands. Discuss any unexpected effects you notice with your doctor.
INACTIVE INGREDIENT
INACTIVE INGREDIENTS:STARCH, CORN
CROSPOVIDONE
D&C YELLOW NO. 10
FD&C BLUE NO. 1
FD&C BLUE NO. 2
FD&C RED NO. 40
GELATIN
LACTOSE MONOHYDRATE
MAGNESIUM STEARATE
CELLULOSE, MICROCRYSTALLINE
SHELLAC
PROPYLENE GLYCOL
FERROSOFERRIC OXIDE
TITANIUM DIOXIDE
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Terazosin HydrochlorideTerazosin Hydrochloride CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!