Taron-C DHA
Taron™-C DHA with Ascorbic Acid Precursors
FULL PRESCRIBING INFORMATION: CONTENTS*
- TARON-C DHA DESCRIPTION
- INDICATIONS
- TARON-C DHA CONTRAINDICATIONS
- WARNING
- WARNING
- PRECAUTIONS
- TARON-C DHA ADVERSE REACTIONS
- OVERDOSAGE
- TARON-C DHA DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- STORAGE
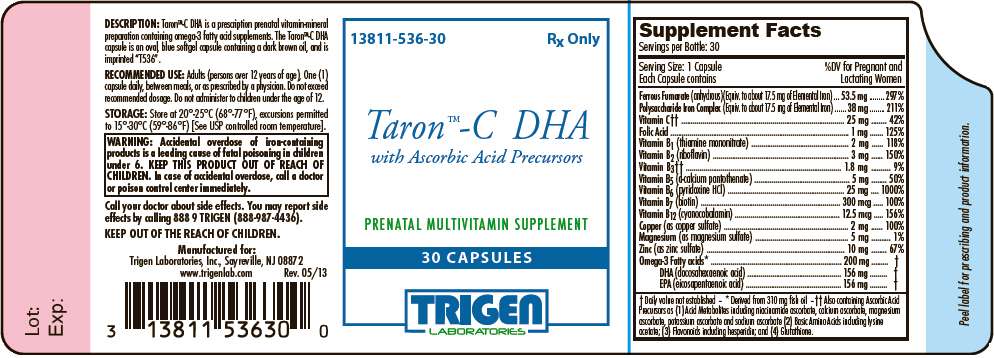
- PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Label
FULL PRESCRIBING INFORMATION
Rx Only
TARON-C DHA DESCRIPTION
Taron™-C DHA is a prescription prenatal vitamin-mineral preparation containing omega-3 fatty acid supplements. The Taron™-C DHA capsule is an oval, blue softgel capsule containing a dark brown oil, and is imprinted with "T536".
| Supplement Facts Servings per Bottle: 30 |
||
|---|---|---|
| Serving Size: 1 Capsule Each Capsule contains |
%DV for Pregnant and Lactating Women |
|
| Ferrous Fumarate (anhydrous)(Equiv. to about 17.5 mg of Elemental Iron) | 53.5 mg | 297% |
| Polysaccharide Iron Complex (Equiv. to about 17.5 mg of Elemental Iron) | 38 mg | 211% |
Vitamin C |
25 mg | 42% |
| Folic Acid | 1 mg | 125% |
| Vitamin B1 (thiamine mononitrate) | 2 mg | 118% |
| Vitamin B2 (riboflavin) | 3 mg | 150% |
Vitamin B3
 |
1.8 mg | 9% |
| Vitamin B5 (d-calcium pantothenate) | 5 mg | 50% |
| Vitamin B6 (pyridoxine HCl) | 25 mg | 1000% |
| Vitamin B7 (biotin) | 300 mcg | 100% |
| Vitamin B12 (cyanocobalamin) | 12.5 mcg | 156% |
| Copper (as copper sulfate) | 2 mg | 100% |
| Magnesium (as magnesium sulfate) | 5 mg | 1% |
| Zinc (as zinc sulfate) | 10 mg | 67% |
|
Omega-3 Fatty acids DHA (docosahexaenoic acid) EPA (eicosapentaenoic acid) |
200 mg 156 mg 156 mg |
   |
Inactive Ingredients: Capsule (Gelatin, Glycerin, Purified Water, Titanium Dioxide, Ethyl Vanillin, FD&C Blue #1), Lecithin, Yellow Beeswax, Natural Creamy Orange Flavor.
INDICATIONS
Taron™-C DHA is a prescription prenatal vitamin-mineral preparation containing omega-3 fatty acid supplements designed to supply nutritional supplementation for women throughout pregnancy and during the postnatal period to lactating and non-lactating mothers. Taron™-C DHA may also be used to improve the nutritional status of women before conception.
TARON-C DHA CONTRAINDICATIONS
Taron™-C DHA is contraindicated in patients with a known hypersensitivity to any of its ingredients, including fish or fish oil; also, all iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis, or hemolytic anemias. Pernicious anemia is a contraindication, as folic acid may obscure its signs and symptoms.
| WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. KEEP THIS PRODUCT OUT OF REACH OF CHILDREN. In case of accidental overdose, call a doctor or poison control center immediately. |
THIS PRODUCT CONTAINS SOY AND FISH OIL.
WARNING
Ingestion of more than 3 grams of omega-3 fatty acids from fish oils per day may have potential antithrombotic effects, including an increased bleeding time and INR (International Normalized Ratio). Omega-3 fatty acids from fish oils (e.g., DHA) should be avoided in patients with inherited or acquired bleeding diatheses, including those taking anticoagulants.
WARNING
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B12 is deficient.
PRECAUTIONS
General
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive.
Pediatric Use
Safety and effectiveness of this product have not been established in pediatric patients.
Geriatric Use
No clinical studies have been performed in patients 65 and older to determine whether older persons respond differently from younger persons. Dosage should always begin at the low end of the dosage scale and should consider that elderly persons may have decreased hepatic, renal, or cardiac function and/or concomitant diseases.
TARON-C DHA ADVERSE REACTIONS
Folic Acid
Allergic sensitization have been reported following both oral and parenteral administration of folic acid.
Ferrous Fumarate
Gastrointestinal disturbances (anorexia, nausea, diarrhea,constipation) occur occasionally, but are usually mild and may subside with continuation of therapy. Although the absorption of iron is best when taken between meals, giving Taron™-C DHA after meals may control occasional G.I. disturbances. Taron™-C DHA is best absorbed when taken at bedtime.
OVERDOSAGE
Iron
Signs and Symptoms
Iron is toxic. Acute overdosage of iron may cause nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other symptoms include pallor and cyanosis, melena, shock, drowsiness, and coma. The estimated overdose of orally ingested iron is 300-mg/kg body weight. When overdoses are ingested by children, severe reactions, including fatalities, have resulted. Taron™-C DHA should be stored beyond the reach of children to prevent against accidental iron poisoning. Keep this product out of the reach of children.
Treatment
For specific therapy, exchange transfusion and chelating agents should be used. For general management, perform gastric lavage with sodium bicarbonate solution or milk. Administer intravenous fluids and electrolytes and use oxygen.
TARON-C DHA DOSAGE AND ADMINISTRATION
Adults (persons over 12 years of age), One (1) capsule daily, between meals, or as prescribed by a physician. Do not exceed recommended dosage. Do not administer to children under the age of 12.
HOW SUPPLIED
Taron™-C DHA is dispensed in child-resistant bottles of 30 capsules.
PRODUCT CODE 13811-536-30
STORAGE
Store at 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°- 86°F). [see USP Controlled Room Temperature].
KEEP OUT OF REACH OF CHILDREN
Rx Only
Call your doctor about side effects. You may report side effects by calling 888 9 TRIGEN (888-987-4436).
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product. There are no implied or explicit claims on therapeutic equivalence.
Manufactured for:
TRIGEN Laboratories, Inc., Sayreville, NJ 08872
www.trigenlab.com
Rev. 05/13
PRINCIPAL DISPLAY PANEL - 30 Capsule Bottle Label
13811-536-30
Rx Only
Taron™-C DHA
with Ascorbic Acid Precursors
PRENATAL MULTIVITAMIN SUPPLEMENT
30 CAPSULES
TRIGEN
LABORATORIES
Taron-C DHAferrous fumarate, IRON, ascorbic acid, folic acid, thiamine mononitrate, riboflavin, Niacin, calcium pantothenate, pyridoxine Hydrochloride, biotin, cyanocobalamin, Cupric Sulfate, magnesium sulfate, zinc sulfate, and Omega-3 fatty acids CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||