Sysco
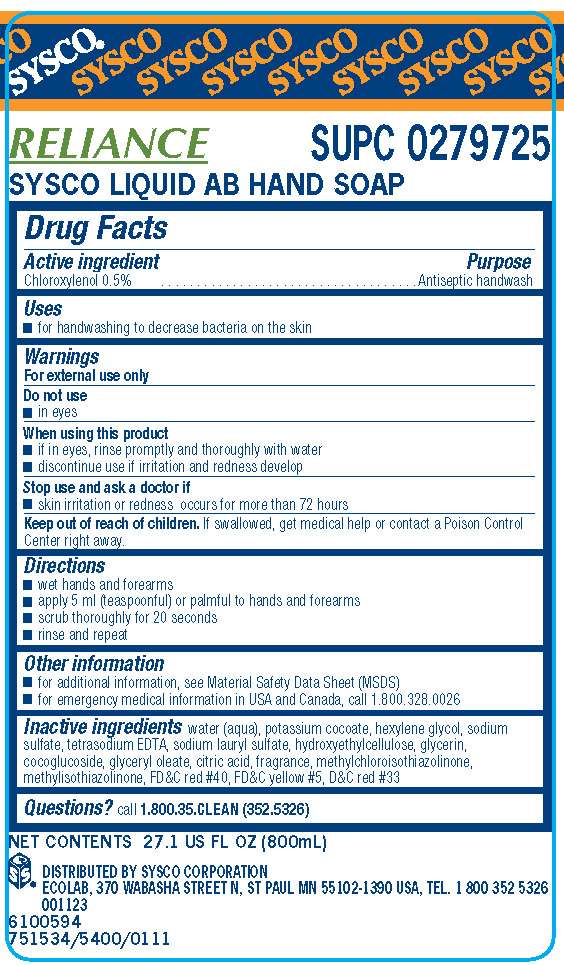
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Sysco Uses
- Warnings
- Directions
- Sysco Other information
- Principal display panel and representative label
FULL PRESCRIBING INFORMATION
Active ingredient
Chloroxylenol 0.5%
Purpose
Antiseptic handwash
Sysco Uses
- for handwashing to decrease bacteria on the skin
Warnings
For external use only
Do not use
- in eyes
When using this product
- if in eyes, rinse promptly and thoroughly with water
- discontinue use if irritation and redness develop
Stop use and ask doctor if
- skin irritation or redness occurs for more than 72 hours
Keep our of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wet hands and forearms
- apply 5 ml (teaspoonful) or palmful to hands and forearms
- scrub thoroughly for 20 seconds
- rinse and repeat
Sysco Other information
- for additional information, see Material Safety Data Sheet (MSDS)
- for emergency medical information in USA and Canada, call 1.800.328.0026
Inactive ingredients water (aqua), potassium cocoate, hexylene glycol, sodium sulfate, tetrasodium EDTA, sodium lauryl sulfate, hydroxyehtylcellulose, glycerin, cocoglucoside, glyceryl oleate, citric acid, fragrance, methylchloroisothiazolinone, methylisothiazolinone, FDC red 40, FDC yellow 5, DC red 33
Questions? call 1.800.37.CLEAN (352.5326)
Principal display panel and representative label
SYSCO
RELIANCE SUPC 0279725
SYSCO LIQUID AB HAND SOAP
NET CONTENTS 27.1 US FL OZ (800mL)
DISTRIBUTED BY SYSCO CORPORATION
ECOLAB, 370 WABASHA STREET N, ST PAUL MN 55102-1390 USA, TEL. 1800 352 5326
001123
65100594
751534/5400/0111
Syscochloroxylenol SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||