SULPHO-LAC MEDICATED
Bradford Soap Works, Inc.
Bradford Soap Works, Inc.
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENT
- INDICATIONS
- WHEN USING THIS PRODUCT
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- PACKAGE IMAGE
FULL PRESCRIBING INFORMATION
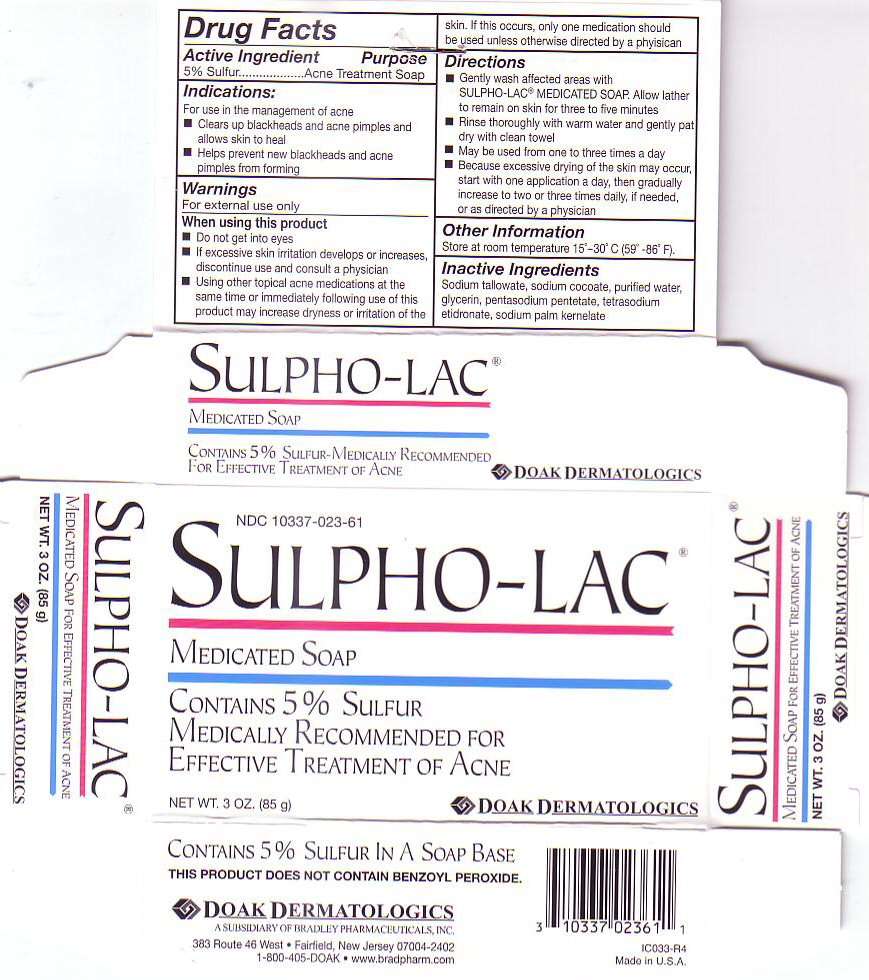
ACTIVE INGREDIENT
PURPOSE
ACNE TREATMENT SOAP
INDICATIONS
- CLEARS UP BLACKHEADS AND ACNE PIMPLES AND ALLOWS SKIN TO HEAL
- HELPS PREVENT NEW BLACKHEADS AND ACNE PIMPLES FROM FORMING
WARNINGS
FOR EXTERNAL USE ONLYWHEN USING THIS PRODUCT
- DO NOT GET INTO EYES
- IF EXCESSIVE SKIN IRRITATION DEVELOPS OR INCREASES, DISCONTINUE USE AND CONSULT A PHYSICIAN
- USING OTHER TOPICAL ACNE MEDICATIONS AT THE SAME TIME OR IMMEDIATELY FOLLOWING USE OF THIS PRODUCT MAY INCREASE DRYNESS OR IRRITATION OF THE SKIN. IF THIS OCCURS ONLY ONE MEDICATION SHOULD BE USED UNLESS OTHERWISE DIRECTED BY A PHYSICAN
DIRECTIONS
- GENTLY WASH AFFECTED AREA WITH SULPHO-LAC MEDICATED SOAP. ALLOW LATHER TO REMAIN ON SKIN FOR THREE TO FIVE MINUTES
- RINSE THOROUGHLY WITH WARM WATER AND GENTLY PAT DRY WITH CLEAN TOWEL
- MAY BE USED FROM ONE TO THREE TIMES A DAY
- BECAUSE EXCESSIVE DRYNESS OF THE SKIN MAY OCCUR, START WITH ONE APPLICATION A DAY, THEN GRADUALLY INCREASE TO TWO OR THREE TIMES DAILY, IF NEEDED, OR AS DIRECTED BY A PHYSICIAN
OTHER INFORMATION
STORE AT ROOM TEMPERATURE 150 - 300 C (590 - 860 F).
INACTIVE INGREDIENTS
SODIUM TALLOWATE, SODIUM COCOATE, PURIFIED WATER, PENTASODIUM PENTETATE, TETRASODIUM ETIDRONATE, SODIUM PALM KERNELATE
PACKAGE IMAGE
SULPHO-LAC MEDICATEDSULFUR SOAP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!