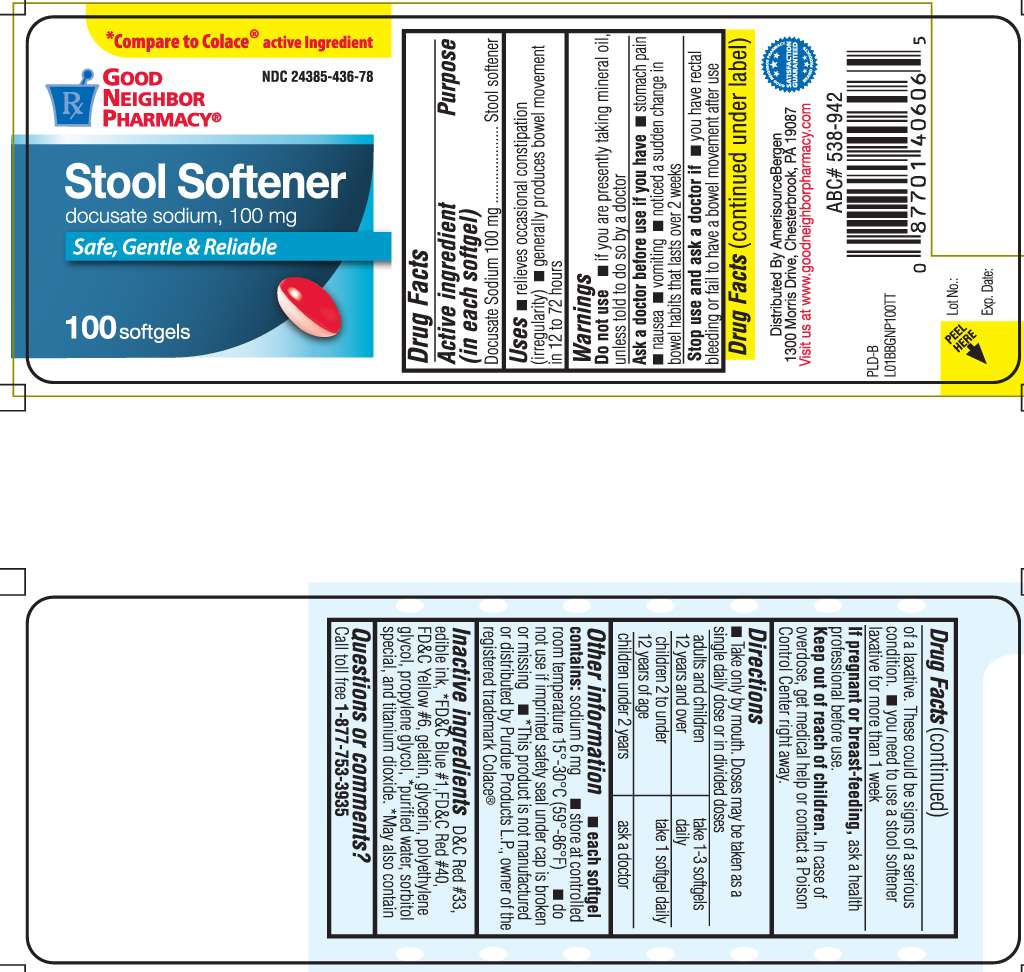
stool softener
Amerisourcebergen Drug Corporation (Good Neighbour Pharmacy)
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Docusate Sodium 100 mg
Stool softener
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
- if you are presently taking mineral oil, unless told to do so by a doctor
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- you need to use a stool softener laxative for more than 1 week
ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away.
- Take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1- 3 softgels daily |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years | ask a doctor |
- each softgel contains: sodium 6 mg
- store at controlled room temperature 15o - 30o C (59o- 86o F)
- do not use if imprinted safety seal under cap is broken or missing
- *This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Colace®
D&C Red #33, edible ink, *FD&C Blue #1, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, *purified water, sorbitol special, and titanium dioxide.
*May also contain
Call toll free 1-877-753-3935
Principal display panel
*compare to colace® active ingredient
STOOL SOFTENER
Docusate Sodium, 100 mg
safe, gentle & reliable
Docusate Sodium 100 mg
stool softenerDOCUSATE SODIUM CAPSULE, LIQUID FILLED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!