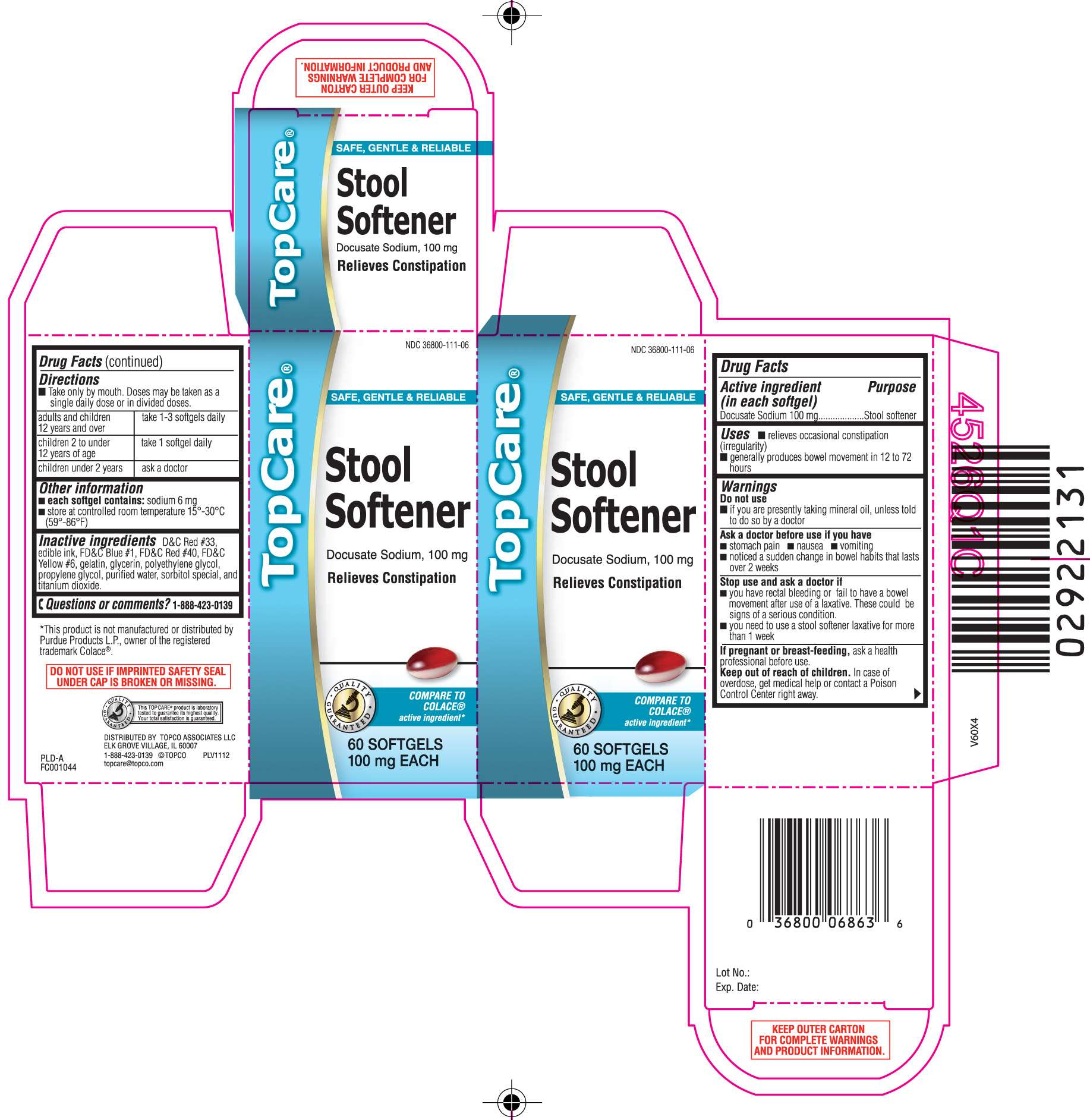
STOOL SOFTENER
TOP CARE (Topco Associates LLC)
P and L Development of New York Corporation
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each softgel)
- Purpose
- STOOL SOFTENER Uses
- Warnings
- Directions
- STOOL SOFTENER Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
- Package Label
FULL PRESCRIBING INFORMATION
Active ingredient (in each softgel)
Docusate Sodium 100 mg
Purpose
Stool softener
STOOL SOFTENER Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 12 to 72 hours
Warnings
Do not use
- if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if
- you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
- you need to use a stool softener laxative for more than 1 week
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Take only by mouth. Doses may be taken as a single daily dose or in divided doses.
| adults and children 12 years and over | take 1-3 softgels daily |
| children 2 to under 12 years of age | take 1 softgel daily |
| children under 2 years | ask a doctor |
STOOL SOFTENER Other information
- each softgel contains: sodium 6 mg
- store at controlled room temperature 15°-30°C (59°-86°F)
Inactive ingredients
D&C Red #33, edible ink, FD&C Blue #1, FD&C Red #40, FD&C Yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, purified water, sorbitol special, and titanium dioxide
Questions or comments?
1-888-423-0139
Principal Display Panel
SAFE, GENTLE & RELIABLE
Stool Softener
Docusate Sodium, 100 mg
Relieves Constipation
COMPARE TO COLACE® active ingreident*
SOFTGELS 100 mg EACH
*This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Colace®.
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
DISTRIBUTED BY TOPCO ASSOCIATES LLC
ELK GROVE VILLAGE, IL 60007
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION.
Package Label
STOOL SOFTENERDOCUSATE SODIUM CAPSULE, LIQUID FILLED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||