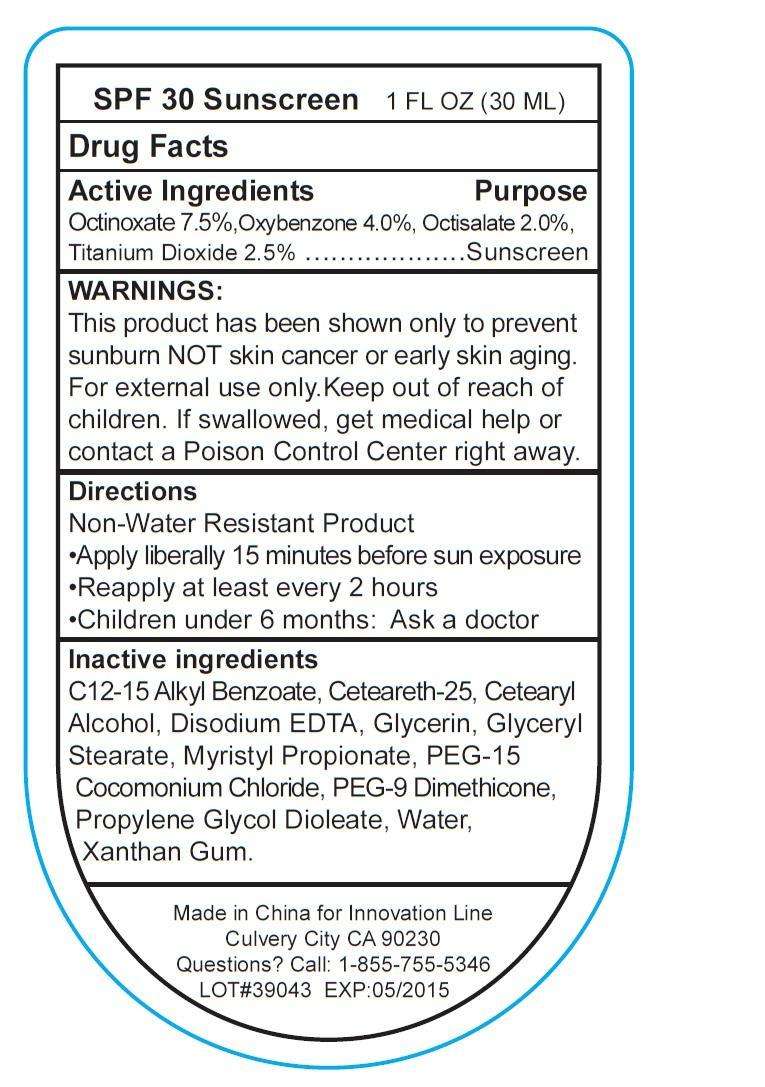
SPF 30 Sunscreen
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active Ingredients
Octinoxate 7.5 %
Oxybenzone 4.0 %
Octisalate 2.0 %
Titanium Dioxide 2.5%
Purpose
Purpose
Sunscreen
Keep out of reach of children, If swallowed get medical help or contact a Poison Control Center.
Uses
Directions
- Non-Water Resistant Product.
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Children under 6 months : Ask a doctor
Warnings:
This product has been shown only to prevent sunburn NOT skin cancer or early skin aging.
For external use only. Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Dosage and Administration
- Apply liberally 15 minutes before sun exposure.
- Reapply at least every 2 hours.
- Children under 6 months : Ask a doctor
Inactive Ingredients
C12-15 Alkyl Benzoate, Ceteareth-25, Cetearyl Alcohol, Disodium EDTA, Glycerin, Glyceryl
Stearate, Myristyl Propionate, PEG-15 Cocomonium Chloride, PEG-9 Dimethicone,
Propylene Glycol Dioleate, Water, Xanthan Gum.
SPF 30 SunscreenOctinoxate, Oxybenzone, Octisalate, Titanium Dioxide LOTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||