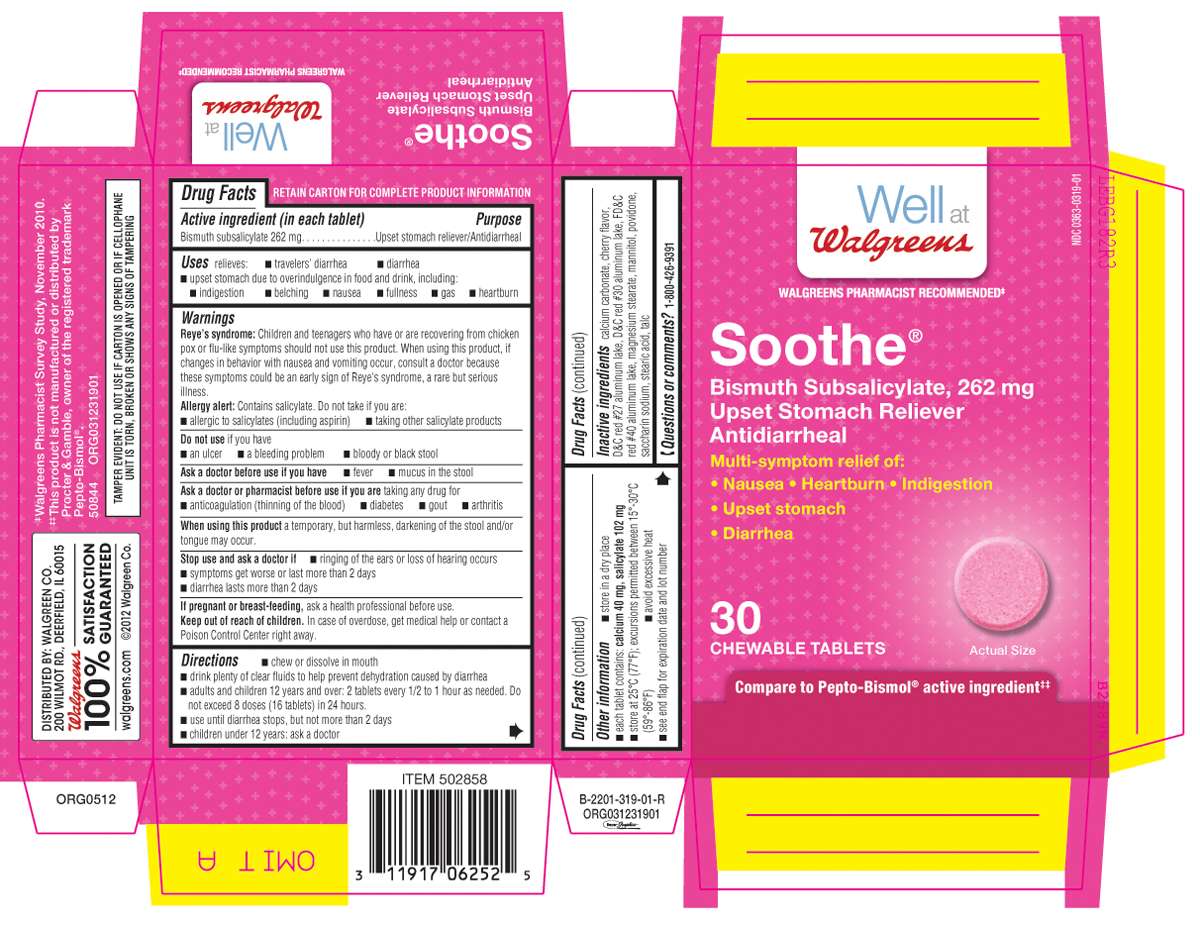
Soothe
Walgreens 44-319
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- Soothe Uses
- Warnings
- Directions
- Soothe Other information
- Inactive ingredients
- Questions or comments?
- Product Packaging
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Bismuth subsalicylate 262 mg
Purpose
Upset stomach reliever/Antidiarrheal
Soothe Uses
relieves:
- traveler's diarrhea
- diarrhea
- upset stomach due to overindulgence in food and drink, including:
- indigestion
- belching
- nausea
- fullness
- gas
- heartburn
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye"s syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are:
-
allergic to salicylates (including aspirin)
-
taking other salicylate products
Do not use
if you have
-
an ulcer
-
a bleeding problem
-
bloody or black stool
Ask a doctor before use if
-
fever
-
mucus in the stool
Ask a doctor or pharmacist before use if you are
taking any drug for
-
anticoagulation (thinning of the blood)
-
diabetes
-
gout
-
arthritis
When using this product
a temporary, but harmless, darkening of the stool and/or tongue may occur.
Stop use and ask a doctor if
- ringing of the ears or loss of hearing occurs
- symptoms get worse or lasts more than 2 days
- diarrhea lasts more than 2 days
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
-
chew or dissolve in mouth
-
drink plenty of clear fluids to help prevent dehydration caused by diarrhea
-
adults and children 12 years and over: 2 tablets every 1/2 to 1 hour as needed. Do not exceed 8 doses (16 tablets) in 24 hours.
-
use until diarrhea stops, but not more than 2 days
-
children under 12 years: ask a doctor
Soothe Other information
-
store in a dry place
-
each tablet contains: calcium 40 mg, salicylate 102 mg
-
store at 25ºC (77ºF); excursions permitted between 15°-30°C (59°-86°F)
-
avoid excessive heat
-
see end flap for expiration date and lot number
Inactive ingredients
calcium carbonate, cherry flavor, D&C red #27 aluminum lake, D&C red #30 aluminum lake, FD&C red #40 aluminum lake, magnesium stearate, mannitol, povidone, saccharin sodium, stearic acid, talc
Questions or comments?
1-800-426-9391
Product Packaging
Well at
Walgreens
WALGREENS PHARMACIST RECOMMENDEDǂ
NDC 0363-0319-01
Soothe®
Bismuth Subsalicylate, 262 mg
Upset Stomach Reliever
Antidiarrheal
Multi-symptom relief of:
• Nausea • Heartburn • Indigestion
• Upset stomach
• Diarrhea
30
CHEWABLE TABLETS
Compare to Pepto-Bismol® active ingredientǂǂ
ǂWalgreens Pharmacist Survey Study, November 2010.
ǂǂThis product is not manufactured or distributed by Procter & Gamble,owner of the registered trademark Pepto-Bismol®.
50844 ORG031231901
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
SootheBISMUTH SUBSALICYLATE TABLET, CHEWABLE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||