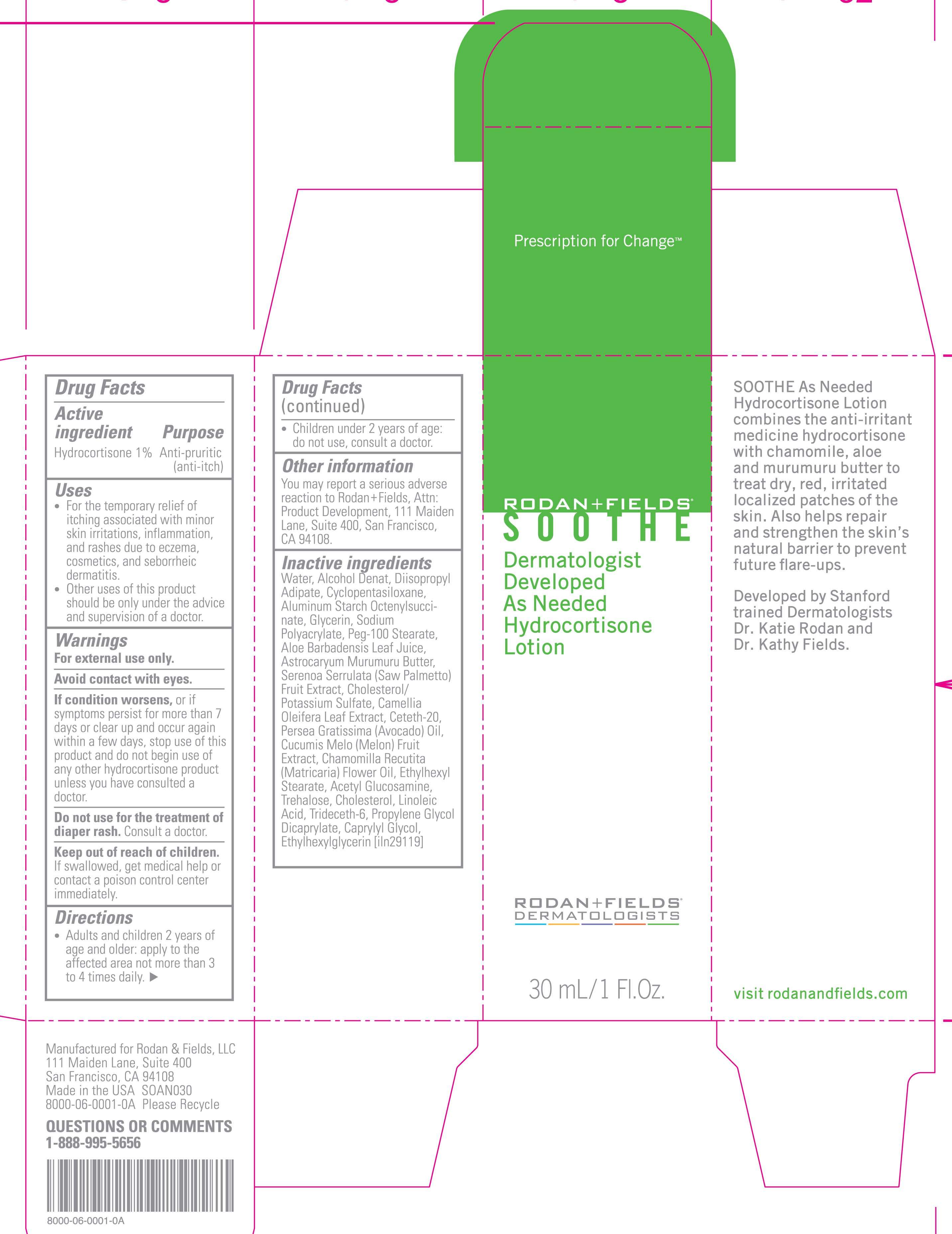
Soothe
Rodan & Fields, LLC.
COSMETIC ENTERPRISES LTD
Rodan And Fields Hydrocortisone Lotion
FULL PRESCRIBING INFORMATION
Active ingredient
HYDROCORTISONE 1%
Purpose
Anti- Pruritic ( Anti- Itch)
Keep out of reach of children.
if swallowed, get medical help or contact a poison control center immediately.
Uses
adults and children 2 years of age and older: apply to the effected area not more than 3 to 4 times daily.
children under 2 years of age: do not use, consult a doctor.
Water, Alcohol Denat, Diisopropyl Adipate, Cyclopentasiloxane, Aluminum Starch Octenylsuccinate, Glycerin, Sodim Polyacrylate, Peg-100 Stearate, Aloe Barbadensis Leaf Juice, Astrocaryum Murumuru Butter, Serenoa Serrulata ( Saw Palmetto) Fruit Extract, Cholesterol/ Potassium Sulfate, Camellia Oleifera Leaf Extract, Ceteth-20, Persea Gratissima ( Avocado) Oil, Cucumis Melo ( Melon) Fruit Extract, Chamomilla Recutita ( Matricaria) Flower Oil, Ethylhexyl Stearate, Acetyl Glucosamine, Trehalose, Cholesterol, Linoleic Acid, Trideceth-6, Propylene Glycol, Dicaprylate, Caprylyl Glycol, Ethylhexylglycerin
Rodan and Fields
Soothe
Dermatologist Developed as needed
Hydrocortisone Lotion
30 mL/ 1 Fl. Oz.

SootheHYDROCORTISONE LOTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||