Sodium Fluoride F 18
Triad Isotopes, Inc.
Triad Isotopes, Inc.
Sodium Fluoride F 18 Injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- SODIUM FLUORIDE F 18 DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS
- SODIUM FLUORIDE F 18 CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SODIUM FLUORIDE F 18 ADVERSE REACTIONS
- OVERDOSAGE
- SODIUM FLUORIDE F 18 DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Diagnostic - For Intravenous Administration
SODIUM FLUORIDE F 18 DESCRIPTION
Sodium Fluoride F 18 Injection is a positron emitting radiopharmaceutical, containing no-carrier added, radioactive fluoride F 18 that is used for diagnostic purposes in conjunction with positron emission tomography (PET) imaging and is administered by intravenous injection.
The active ingredient, sodium fluoride F 18, has the molecular formula of Na18F with a molecular weight of 40.99, and has the following chemical structure:
Na+ 18F –
Sodium Fluoride F 18 Injection is provided as a ready to use sterile, pyrogen-free, clear and colorless solution. Each mL of the solution contains between 0.185 GBq to 17.575 GBq (5 to 475 mCi) fluoride F 18, at the end of synthesis (EOS) reference time, and may contain up to 1.8 mg of sodium chloride. The pH of the solution is between 4.5 to 8.0. The solution does not contain any preservatives. The only known source of nonradioactive fluorine ion present is that found in the distilled water and saline solutions used in preparing this product.
Physical Characteristics
Fluorine F 18 decays by positron (β +) emission and has a half-life of 109.8 minutes. The principal photons useful for diagnostic imaging are the 511 keV gamma photons, resulting from the interaction of the emitted positron with an electron (Table 1).
| Radiation/Emission | % per Disintegration | Mean Energy |
|---|---|---|
| Positron (β +) | 96.73 | 249.8 keV |
| Gamma (±) |
193.46 | 511.0 keV |
From: Kocher, D.C. "Radioactive Decay Tables" DOE/TIC-I 1026, 89 (1981).
External Radiation
The specific gamma ray constant for Fluorine F 18 is 6.0 R/hr/mCi (1.35 × 10 -6 Gy/hr/kBq) at 1cm. The half-value layer (HVL) for the 511 keV photons is 4.1 mm lead (Pb). A range of values for the attenuation of radiation results from the interposition of various thickness of Pb. The range of attenuation coefficients for this radionuclide is shown in Table 2. For example, the interposition of an 8.3 mm thickness of Pb with a coefficient of attenuation of 0.25 will decrease the external radiation by 75%.
| Shield Thickness (Pb) mm | Coefficient of Attenuation |
|---|---|
| 0 | 0.00 |
| 4.1 | 0.50 |
| 8.3 | 0.25 |
| 13.2 | 0.10 |
| 26.4 | 0.01 |
| 52.8 | 0.001 |
For use in correcting for physical decay of this radionuclide, the fractions remaining at selected intervals after calibration are shown in Table 3.
| Minutes | Fraction Remaining |
|---|---|
| 0 |
1.00 |
| 15 | 0.909 |
| 30 | 0.826 |
| 60 | 0.683 |
| 110 | 0.500 |
| 220 | 0.250 |
| 440 | 0.060 |
CLINICAL PHARMACOLOGY
General
Fluorine F 18 ions normally accumulate in the skeleton in an even fashion, with greater deposition in the axial skeleton (e.g., vertebrae and pelvis) than in the appendicular skeleton and greater deposition in the bones around joints than in the shafts of long bones.
Pharmacodynamics
Increased fluorine F 18 ion deposition around joints can occur in arthritis or following trauma; increased deposition has also been documented in bone around fracture sites, in osteomyelities, fibrous dysplasia, spondylitis tuberculosa, Paget's disease, hyperstosis frontalis interna, myositis, ossificans, and in rapidly growing epiphyses. The tendency for fluorine F 18 ions to accumulate in the vicinity of primary and metastatic malignancy in bone has proven clinically useful in detection of such lesions (3-10).
Pharmacokinetics
Following intravenous administration, Sodium Fluoride F 18 Injection provides fluorine F 18 ions that rapidly equilibrate, primarily within the extracellular fluid space. These fluorine F 18 ions are then rapidly cleared by bone deposition and by excretion into the urine.
Distribution
Deposition of the fluorine F 18 ions in bone appears to be primarily a function of blood flow to the bone and the efficiency of the bone in extracting the fluorine F 18 ions from the blood perfusing the bone (1). Fluorine F 18 ions do not appear to be bound to serum proteins.
Elimination
In patients with normal renal function, 20% or more of the fluorine F 18 ions are cleared from the body in the urine within the first 2 hours after intravenous administration (3). Subsequently, small amounts of fluorine 18 ions continue to be excreted in the urine further diminishing the radioactivity of the fluorine F 18 ions in soft tissues of the body. (See Pharmacokinetics section)
The clearance of fluorine F 18 ions from the blood is rapid (2). Fluorine F 18 ions are rapidly eliminated via the renal system.
Drug-Drug Interactions
Drug-drug interactions with Sodium Fluoride F 18 Injection have not been studied.
INDICATIONS
Sodium Fluoride F 18 Injection is used as a bone imaging agent to define areas of altered osteogenic activity.
SODIUM FLUORIDE F 18 CONTRAINDICATIONS
None known.
WARNINGS
None known.
PRECAUTIONS
General
Radiopharmaceuticals should be used only by personnel (e.g., physicians or radiopharmacists) who are qualified by specific training in the safe use and handling of radioactive drugs and materials. (See Drug Handling section.) In the use of any radiopharmaceutical, care should be taken to ensure minimum radiation exposure to the patient and all personnel involved in the procedure by using the smallest dose of radioactivity consistent with safety and relative value of diagnostic information.
As with other injectable drug products, allergic reactions and anaphylaxis may occur. Emergency resuscitation equipment and personnel should be immediately available.
Information for Patients
To minimize the radiation-absorbed dose to the bladder, adequate hydration should be encouraged to stimulate frequent voiding during the first few hours after intravenous administration of Sodium Fluoride F 18 Injection. This may be achieved by having patients drink at least an 8 oz. glass of water prior to drug administration. To help protect themselves and others in their environment, patients should take the following precautions for 12 hours after injection: whenever possible a toilet should be used and should be flushed several times after each use; wash hands thoroughly after each voiding or fecal elimination. If blood, urine, or feces soil clothing, the clothing should be washed separately.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies with Sodium Fluoride F 18 Injection have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with Sodium Fluoride F 18 Injection. It is not known whether Sodium Fluoride F 18 Injection can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Therefore, Sodium Fluoride F 18 Injection should not be administered to a pregnant woman unless the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
The effects of Sodium Fluoride F 18 Injection on human breast milk are unknown. Because many drugs are excreted in human milk, caution should be exercised when Sodium Fluoride F 18 Injection is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of Sodium Fluoride F 18 Injection has not been established in pediatric patients. Like other bone imaging agents, Sodium Fluoride F 18 Injection is known to localize in the rapidly growing epiphyses in developing long bones. (See Clinical Pharmacology section.)
SODIUM FLUORIDE F 18 ADVERSE REACTIONS
Fluorine ions are a normal body constituent. The amount of fluorine ions in Sodium Fluoride F 18 Injection at the indicated dose has minimal effect on normal human physiology.
When Sodium Fluoride F 18 Injection was approved for marketing in 1972, no adverse reactions were noted in over 400 patient studies reported in the medical literature. In a 1999 review of the published literature, publicly available reference sources and adverse drug reaction reporting systems indicated that adverse reactions have not been reported for Sodium Fluoride F 18 Injection. However, patients should be appropriately monitored for adverse drug reactions, and such reactions should be reported to FDA, if they occur.
OVERDOSAGE
Overdoses of Sodium Fluoride F 18 Injection have not been reported. (See also Radiation Dosimetry section.)
SODIUM FLUORIDE F 18 DOSAGE AND ADMINISTRATION
The recommended dose of Sodium Fluoride F 18 Injection is 0.185 to 0.37 GBq (5 to 10 mCi), as an intravenous injection.
The dose of Sodium Fluoride F 18 Injection used in a given patient should be minimized consistent with the objectives of the study, and the nature of the radiation detection devices employed. (See Radiation Dosimetry section.)
The final dose for the patient should be calculated using proper decay factors from the time of the end of synthesis (EOS), and measured by a suitable radioactivity calibration system before administration. (See decay factors in Table 3.)
Patient Preparation
The patient should be instructed to ingest copious amounts of fluid immediately prior and subsequent to the administration of Sodium Fluoride F 18 Injection. The patient should void one-half hour after administration of Sodium Fluoride F 18 Injection and as frequently thereafter as possible. The patient should be instructed to void immediately prior to imaging the fluorine F 18 radioactivity in the lumbar spine or bony pelvis.
Imaging
Optimally, it is recommended that imaging of Sodium Fluoride F 18 Injection can begin 1 to 2 hours after administration; however, the longer the imaging procedure is delayed, the greater will be the ratio of the activity in bone to that in soft tissue. Voiding immediately prior to imaging the fluorine F 18 biodistribution in the pelvis is recommended to reduce background radioactivity.
Drug Handling
Sodium Fluoride F 18 Injection, like other parenteral drug products, should be inspected visually for particulate matter and discoloration before administration, whenever solution and container permit. Sodium Fluoride F 18 Injection preparations containing particulate matter or discoloration should not be administered. They should be disposed of in a safe manner in compliance with applicable regulations.
Aseptic techniques and effective shielding should be employed in withdrawing doses for administration to patients. Waterproof gloves and effective shielding should be worn when handling the product.
The contents of each vial are sterile and non-pyrogenic. To maintain sterility, aseptic technique must be used during all operations involved in the manipulation and administration of Sodium Fluoride F 18 Injection.
As with any other radioactive material, appropriate shielding should be used to avoid unnecessary radiation exposure to the patient, occupational workers, and other persons.
Sodium Fluoride F 18 Injection, like other radioactive drugs, must be handled with care, and appropriate safety measures should be used to minimize radiation exposure to clinical personnel. Care should be taken to minimize exposure to the patient consistent with proper patient management. Radiopharmaceuticals should be used by or under the control of physicians who are qualified by specific training and experience in the safe use and handling of radionuclides, and whose experience and training have been approved by the appropriate governmental agency authorized to license the use of radionuclides.
Radiation Dosimetry
Following are the estimated absorbed radiation doses (rem/mCi) to a human adult (70 kg) from intravenous injection of Sodium Fluoride F 18 Injection. These estimates were calculated based on human data and using the data published by the International Commission on Radiological Protection for Sodium Fluoride F 18 Injection (11). Fluorine F 18 ions decay with a physical halflife of 109.8 minutes. Ninety-seven percent (97%) of the decay results in emission of a positron with a maximum energy of 0.635 MeV, and 3% of the decay results in electron capture with subsequent emission of characteristic X-rays of oxygen. The bone and bone marrow are considered the target and critical organs. The absolute values for absorbed radiation in relation to age have not been established.
| Estimated Radiation Dose | ||
|---|---|---|
| ORGAN | mGy/MBq | rad/mCi |
| Adrenals | 0.0062 | 0.023 |
| Brain | 0.0056 | 0.021 |
| Breasts | 0.0028 | 0.010 |
| Gallbladder wall | 0.0044 | 0.016 |
| LLI wall |
0.012 | 0.043 |
| Small intestine | 0.0066 | 0.025 |
| Stomach | 0.0038 | 0.014 |
| ULI wall |
0.0058 | 0.021 |
| Heart wall | 0.0039 | 0.015 |
| Kidneys | 0.019 | 0.071 |
| Liver | 0.0040 | 0.015 |
| Lungs | 0.0041 | 0.015 |
| Muscle | 0.0060 | 0.022 |
| Ovaries | 0.011 | 0.039 |
| Pancreas | 0.0048 | 0.018 |
| Red marrow | 0.028 | 0.010 |
| Bone surfaces | 0.060 | 0.22 |
| Skin | 0.004 | 0.015 |
| Spleen | 0.0042 | 0.015 |
| Testes | 0.0078 | 0.029 |
| Thymus | 0.0035 | 0.013 |
| Thyroid | 0.0044 | 0.016 |
| Urinary bladder wall | 0.25 | 0.91 |
| Uterus | 0.019 | 0.070 |
| Effective Dose Equivalent | 0.027 mSv/MBq | 0.10 rem/mCi |
Estimates were calculated using phantom of Cristy & Eckerman (Report ORNL/TM-8381/V1 & V7). Models for bone and marrow are adopted from Eckerman (Aspects of dosimetry of radionuclides within the skeleton with particular emphasis on the active marrow, In Fourth International Radiopharmaceutical Dosimetry Symposium; A.T. Schlafke-Stelson and E. E. Watson eds. CONF-851113, Oak Ridge Associated Universities, Oak Ridge, TN 37831, 1986. pp 514-534.). Additional information was obtained from the Radiation Internal Dose Information Center, Oak Ridge Associated Universities, Oak Ridge, TN 37831.
HOW SUPPLIED
Sodium Fluoride F 18 Injection is supplied in 10, 20 or 30 mL vials containing between 0.185 – 17.575 GBq/mL (5 - 475 mCi/mL) of no carrier added sodium [18F] fluoride, at end of synthesis (EOS) reference time.
NDC Code:
10 ml vial NDC 60055-018-10
20 ml vial NDC 60055-018-20
30 ml vial NDC 60055-018-30
This sterile injectable solution is approved by the U.S. Nuclear Regulatory Commission for
distribution to persons licensed to use by-product material identified in
§35.200 to 10 CFR Part 35, to persons who have a similar authorization issued by
an Agreement State, and, outside the United States, to persons authorized by the
appropriate authority.
Storage
Sodium Fluoride F 18 Injection should be stored upright in a lead shielded container at controlled room temperatures.
Storage and disposal of Sodium Fluoride F 18 Injection should be in accordance with the regulations and a general license, or its equivalent, of an Agreement State or a Licensing State.
Expiration Date and Time
The expiration date and time are provided on the container label. Sodium Fluoride F18 Injection should be used within 12 hours from the time of the end of synthesis (EOS) reference time.
Caution: Rx ONLY
| Manufactured by: | Triad Isotopes, Inc. 5215 Langford Park Dr. Norcross, GA, 30071 |
| Distributed by: | Triad Isotopes, Inc. 5215 Langford Park Dr. Norcross, GA, 30071 |
REFERENCES
- D. Van Dyke, H. O. Anger, Y. Yano, and C. Bozzini, "Bone Blood Flow Shown with 18F and the Positron Camera." Am. J. Physiology 209, 64 (1965).
- D.A. Weber, E.J. Greenberg, A. Dimich, P.J. Kenny, E.O. Rothschild, W.P.L. Meyers and J.S. Laughlin, "Kinetics of Radionuclides used for Bone Studies," J. Nuclear Med. 10, 8 (1969).
- C.L. Harmen, J.E. Burns, A. Sams and M. Spittle, "The Value of 18F for Scanning Bone Tumors," Clin. Radio. 20, 204 (1969).
- H.J. Dworkin and E.V. Filmanowicz, "Radiofluorine Photoscanning of Bone for Reticulum Cell Saracoma: Early Detection of Bone Involvement." J. Amer. Med. Assoc. 198, 985 (1966).
- H.G. Kampffmeyer, H. Dworkin, E.A. Carr, and F.E. Bull, "Effect of Drug Therapy on the Uptake of Radioactive Fluorine by Osseous Metastases." Clin. Pharmacol. Ther. 8, 657 (1967).
- R. Spencer, R. Herbert, M.W. Rish, and W.A. Little, "Bone Scanning with 85Sr and 18F. Physical and Radiopharmacuetical Considerations and Clinical Experience in 50 Cases." Brit. J. Radiol. 40, 641 (1967).
- L.R. Holsti and L.K. Patomake, "18F Scanning of Primary and Metastatic Bone Tumors." Ann. Med. Intern. Fenn. 56, 131 (1967).
- R.J. French and V.R. McReady, "Use of 18F for Bone Scanning," Brit. J. Radiol. 40, 655 (1967).
- N.F. Moon, H.J. Dworkin and P.D. La Fleur, "The Clinical Use of Sodium Fluoride 18F in Bone Photoscanning," J. Amer. Med. Assoc. 204, 974 (1968).
- P. Ronai, H.S. Winchell and H.O. Anger, "Skeletal Survey for Metastatic Tumors of Bone Using 18F and 85Sr with Scintillation Camera and Whole Body Scanner," J. Nuclear Med. 9, 517 (1968).
- ICRP Publication 53, Volume 18, No. l-4, 73 - 74 (1987).
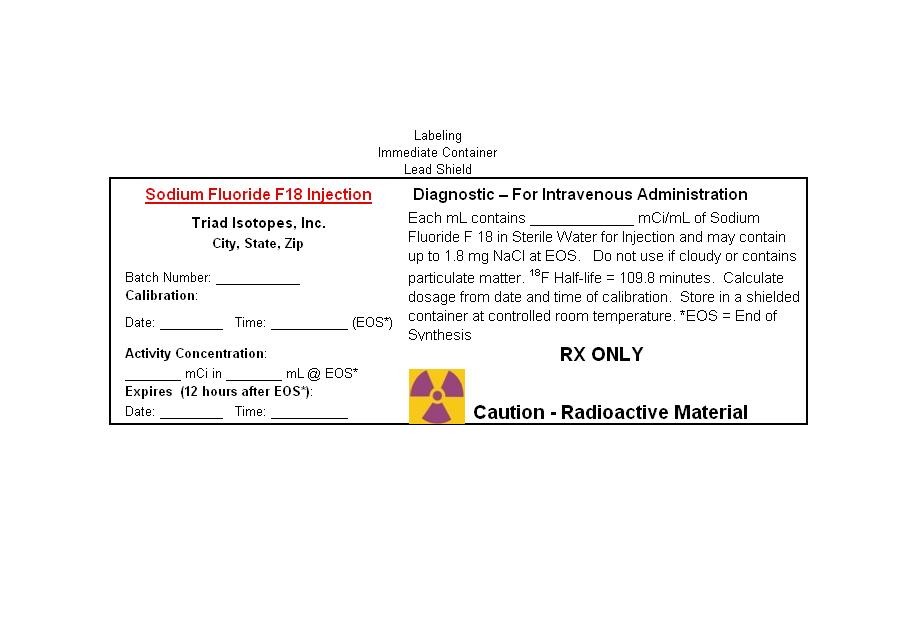
PRINCIPAL DISPLAY PANEL
LABELING
IMMEDIATE CONTAINER
VIAL/LEAD PIG
Sodium Fluoride F 18 Injection
Diagnostic-For Intravenous Administration
| Sodium Fluoride F 18 Injection |
|
|
Diagnostic -- For Intravenous Administration |
| Triad Isotopes Inc |
|
|
Each mL contains___________mCi/mL of Sodium Fluoride |
| City, State, Zip |
|
|
F-18 in Sterile Water for Injection and may contain up to |
| Batch Number: ______________ |
|
|
1.8 mg. of NaCl at EOS. |
| Calibration: | |||
| Date__________Time________(EOS*) |
|
|
Do not use if cloudy or contains particulate matter. The 18F Half- |
| Activity Concentration: |
|
|
life = 109.8 minutes. Calculate dosage from date and time of |
| ________mCi in ________mL @ EOS* |
|
|
calibration. Store in a shielded container at controlled room |
| Expires (12 hours after EOS*) |
|
|
temperature. *EOS = End of Synthesis. RX ONLY |
| Date:_________Time_________ |
|
|
|
|
|
|
|
Caution: Radioactive Material |
Sodium Fluoride F 18Sodium Fluoride F-18 INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||