smart sense cold and allergy
Kmart Corporation Cold & Allergy Elixir Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each 5 mL teaspoonful)
- Purpose
- smart sense cold and allergy Uses
- Warnings
- Directions
- smart sense cold and allergy Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each 5 mL teaspoonful)
Brompheniramine maleate, USP 1 mg
Phenylephrine HCl, USP 2.5 mg
Purpose
Antihistamine
Nasal decongestant
smart sense cold and allergy Uses
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- temporarily relieves these symptoms due to hay fever (allergic rhinitis):
- sneezing
- itching of the nose or throat
- runny nose
- itchy, watery eyes
- temporarily restores freer breathing through the nose
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- to make a child sleepy
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers
When using this product
- do not use more than directed
- drowsiness may occur
- avoid alcoholic beverages
- alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- symptoms do not get better within 7 days or are accompanied by fever
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not take more than 6 doses in any 24-hour period
| age | dose |
| adults and children 12 years and over | 4 tsp every 4-6 hours |
| children 6 to under 12 years | 2 tsp every 4-6 hours |
| children 4 to under 6 years | do not use unless directed by a doctor |
| children under 4 years | do not use |
Other information
- each teaspoon contains: sodium 2 mg
- store at 20°-25°C (68°-77°F)
- not a USP elixir
Inactive ingredients
citric acid, edetate disodium, FD&C blue no. 1, FD&C red no. 40, flavor, glycerin, propylene glycol, purified water, saccharin sodium, sodium benzoate, sorbitol
Questions or comments?
1-800-719-9260
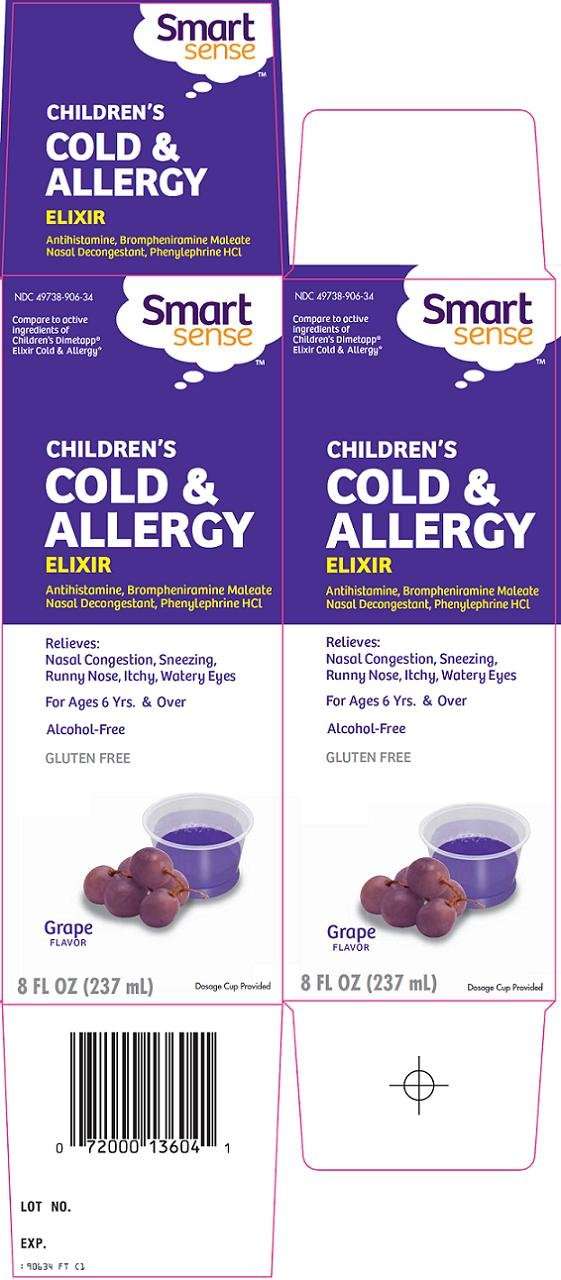
Principal Display Panel
Compare to active ingredients of Children's Dimetapp® Elixir Cold & Allergy
Children’s
Cold & Allergy
Elixir
Antihistamine, Brompheniramine Maleate
Nasal Decongestant, Phenylephrine HCl
Relieves:
Nasal Congestion, Sneezing, Runny Nose, Itchy, Watery Eyes
For Ages 6 yrs. & Over
Alcohol-Free
Gluten Free
Grape Flavor
Dosage Cup Provided
Cold & Allergy Elixir Carton Image 1
Cold & Allergy Elixir Carton Image 2
smart sense cold and allergyphenylephrine hcl, brompheniramine maleate LIQUID
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!