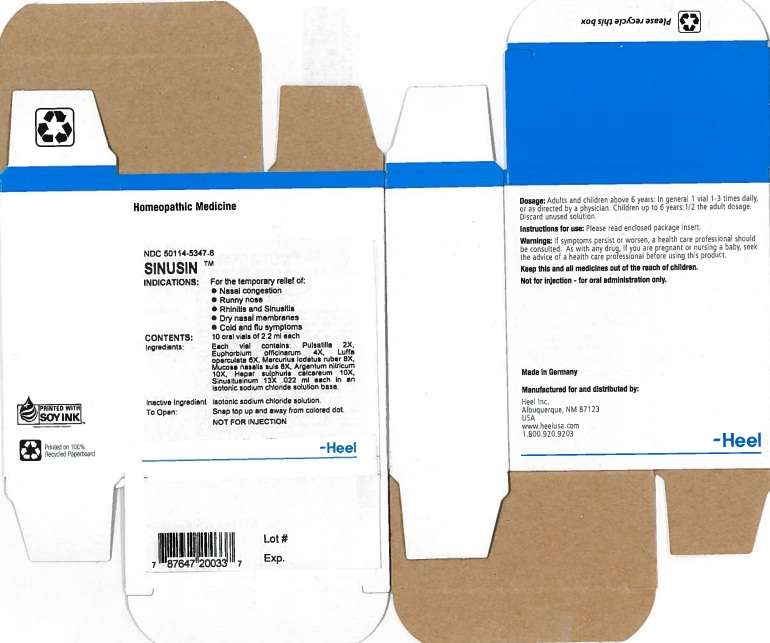
Sinusin
Sinusin 2.2ml Oral Vial
FULL PRESCRIBING INFORMATION: CONTENTS*
- KEEP OUT OF REACH OF CHILDREN
- SINUSIN INDICATIONS AND USAGE
- WARNINGS
- SINUSIN DOSAGE AND ADMINISTRATION
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
- PURPOSE
FULL PRESCRIBING INFORMATION
KEEP OUT OF REACH OF CHILDREN
Keep this ands all medicines out of the reach of children.
SINUSIN INDICATIONS AND USAGE
For the temporary relief of:
- Nasal congestion
- Runny Nose
- Rhinitis and Sinusitis
- Dry nasal membranes
- Cold and flu symptoms
WARNINGS
Warnings: If symptoms persist or worsen, a health care professional should be consulted. As with any drug, if you are pregnant or nursing a baby, seek the advise of a health care professional before using this product.
SINUSIN DOSAGE AND ADMINISTRATION
Adults and children above 6 years: In general 1 vial 1-3 times daily, or as directed by a physician. Children up to 6 years: 1/2 the adult dosage. Discard unused solution.
ACTIVE INGREDIENTS
Each vial contains: Pulsatilla 2X, Euphorbium officinarum 4X, Luffa operculata 6X, Mercurius iodatus ruber 8X, Mucosa nasalis suis 8X, Argentum nitricum 10X, Hepar sulphuris calcareum 10X, Sinusitusinum 13X .022 ml each in an isotonic sodium chloride solution base.
INACTIVE INGREDIENTS
Isotonic sodium chloride solution.
PURPOSE
SinusinPULSATILLA VULGARIS and EUPHORBIA RESINIFERA RESIN and LUFFA OPSILVER NITRATEERCULATA FRUIT and MERCURIC IODIDE and SUS SCROFA NASAL MUCOSA and SILVER NITRATE and CALCIUM SULFIDE and SINUSITISINUM SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||