Simvastatin
Lake Erie Medical & Surgical Supply DBA Quality Care Products LLC
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use simvastatin safely and effectively. See full prescribing information for simvastatin tablets, USP. Simvastatin Tablets, USP Initial U.S. Approval: 1991 RECENT MAJOR CHANGES(2.1)(2.2)(2.3)(2.4)(2.7)(4)(5.1)(5.2)INDICATIONS AND USAGE Reduce the risk of total mortality by reducing CHD deaths and reduce the risk of non-fatal myocardial infarction, stroke, and the need for revascularization procedures in patients at high risk of coronary events. (1.1) Reduce elevated total-C, LDL-C, Apo B, TG and increase HDL-C in patients with primary hyperlipidemia (heterozygous familial and nonfamilial) and mixed dyslipidemia. (1.2) Reduce elevated TG in patients with hypertriglyceridemia and reduce TG and VLDL-C in patients with primary dysbetalipoproteinemia. (1.2) Reduce total-C and LDL-C in adult patients with homozygous familial hypercholesterolemia. (1.2) Reduce elevated total-C, LDL-C, and Apo B in boys and postmenarchal girls, 10 to 17 years of age with heterozygous familial hypercholesterolemia after failing an adequate trial of diet therapy. (1.2, 1.3) (1.4)DOSAGE AND ADMINISTRATION Dose range is 5 to 40 mg/day. (2.1) Recommended usual starting dose is 10 or 20 mg once a day in the evening. (2.1) Recommended starting dose for patients at high risk of CHD is 40 mg/day. (2.1) Due to the increased risk of myopathy, including rhabdomyolysis, use of the 80 mg dose of simvastatin tablets should be restricted to patients who have been taking simvastatin 80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity. (2.2) Patients who are currently tolerating the 80 mg dose of simvastatin tablets who need to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin should be switched to an alternative statin with less potential for the drug-drug interaction. (2.2) Due to the increased risk of myopathy, including rhabdomyolysis, associated with the 80 mg dose of simvastatin tablets, patients unable to achieve their LDL-C goal utilizing the 40 mg dose of simvastatin tablets should not be titrated to the 80 mg dose, but should be placed on alternative LDL-C-lowering treatment(s) that provides greater LDL-C lowering. (2.2) Adolescents (10 to 17 years of age) with HeFH: starting dose is 10 mg/day; maximum recommended dose is 40 mg/day. (2.5) DOSAGE FORMS AND STRENGTHSTablets: 5 mg; 10 mg; 20 mg; 40 mg; 80 mg (3) CONTRAINDICATIONS Concomitant administration of strong CYP3A4 inhibitors. (4, 5.1) Concomitant administration of gemfibrozil, cyclosporine, or danazol. (4, 5.1) Hypersensitivity to any component of this medication. (4, 6.2) Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels. (4, 5.2) Women who are pregnant or may become pregnant. (4, 8.1) Nursing mothers. (4, 8.3) WARNINGS AND PRECAUTIONS Patients should be advised of the increased risk of myopathy including rhabdomyolysis with the 80 mg dose. (5.1) Skeletal muscle effects (e.g., myopathy and rhabdomyolysis): Risks increase with higher doses and concomitant use of certain medicines. Predisposing factors include advanced age (≥65), female gender, uncontrolled hypothyroidism, and renal impairment. (4, 5.1, 8.5, 8.6) Patients should be advised to report promptly any symptoms of myopathy. Simvastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected. See Drug Interaction table. (5.1) Liver enzyme abnormalities and monitoring: Persistent elevations in hepatic transaminase can occur. Monitor liver enzymes before and during treatment. (5.2) Side Effects(6.1) To report SUSPECTED ADVERSE REACTIONS, contact Aurobindo Pharma USA, Inc. at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS Drug Interactions Associated with Increased Risk of Myopathy/Rhabdomyolysis (2.3, 4, 5.1, 7.1, 7.2, 7.3, 12.3) Interacting Agents Prescribing Recommendations Itraconazole, ketoconazole, posaconazole, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, nefazodone, gemfibrozil, cyclosporine, danazol Contraindicated with simvastatin Amiodarone, verapamil, diltiazem Do not exceed 10 mg simvastatin daily Amlodipine, ranolazine Do not exceed 20 mg simvastatin daily Grapefruit juice Avoid large quantities of grapefruit juice (>1 quart daily) Coumarin anticoagulants: Concomitant use with simvastatin prolongs INR. Achieve stable INR prior to starting simvastatin. Monitor INR frequently until stable upon initiation or alteration of simvastatin therapy. (7.6) USE IN SPECIFIC POPULATIONS Severe renal impairment: Patients should be started at 5 mg/day and be closely monitored. (2.6, 8.6)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 SIMVASTATIN INDICATIONS AND USAGE
- 2 SIMVASTATIN DOSAGE AND ADMINISTRATION
- 2.1 Recommended Dosing
- 2.2 Restricted Dosing for 80 mg
- 2.3 Coadministration with Other Drugs
- 2.4 Patients with Homozygous Familial Hypercholesterolemia
- 2.5 Adolescents (10 to 17 years of age) with Heterozygous Familial Hypercholesterolemia
- 2.6 Patients with Renal Impairment
- 2.7 Chinese Patients Taking Lipid-Modifying Doses (≥1 g/day Niacin) of Niacin-Containing Products
- 3 DOSAGE FORMS AND STRENGTHS
- 4 SIMVASTATIN CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 SIMVASTATIN ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 SIMVASTATIN DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Reductions in Risk of CHD Mortality and Cardiovascular Events
- Reduce the risk of total mortality by reducing CHD deaths.
- Reduce the risk of non-fatal myocardial infarction and stroke.
- Reduce the need for coronary and non-coronary revascularization procedures.
1.2 Hyperlipidemia
- Reduce elevated total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), and triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) in patients with primary hyperlipidemia (Fredrickson type IIa, heterozygous familial and nonfamilial) or mixed dyslipidemia (Fredrickson type IIb).
- Reduce elevated TG in patients with hypertriglyceridemia (Fredrickson type IV hyperlipidemia).
- Reduce elevated TG and VLDL-C in patients with primary dysbetalipoproteinemia (Fredrickson type III hyperlipidemia).
- Reduce total-C and LDL-C in patients with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (e.g., LDL apheresis) or if such treatments are unavailable.
1.3 Adolescent Patients with Heterozygous Familial Hypercholesterolemia (HeFH)
- There is a positive family history of premature cardiovascular disease (CVD) or
- Two or more other CVD risk factors are present in the adolescent patient.
1.4 Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
tablets
2.2 Restricted Dosing for 80 mg
first[see Warnings and Precautions (5.1)]
or
dose
2.3 Coadministration with Other Drugs
Patients taking Amiodarone, Verapamil, or Diltiazem
- The dose of simvastatin tablets should not exceed 10 mg/day [see Warnings and Precautions (5.1), Drug Interactions (7.3), and Clinical Pharmacology (12.3)].
Patients taking Amlodipine or Ranolazine
- The dose of simvastatin tablets should not exceed 20 mg/day [see Warnings and Precautions (5.1), Drug Interactions (7.3), and Clinical Pharmacology (12.3)].
2.4 Patients with Homozygous Familial Hypercholesterolemia
is[see Dosage and Administration, Restricted Dosing for 80 mg (2.2)]
2.5 Adolescents (10 to 17 years of age) with Heterozygous Familial Hypercholesterolemia
1 Clinical Studies (14.2)
1Pediatrics.
2.6 Patients with Renal Impairment
[see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]
2.7 Chinese Patients Taking Lipid-Modifying Doses (≥1 g/day Niacin) of Niacin-Containing Products
Chinese[See Warnings and Precautions (5.1).]
3 DOSAGE FORMS AND STRENGTHS
- Tablets simvastatin 5 mg are yellow colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘15’ on the other side.
- Tablets simvastatin 10 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘01’ on the other side.
- Tablets simvastatin 20 mg are light pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘02’ on the other side.
- Tablets simvastatin 40 mg are pink colored, round shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘03’ on the other side.
- Tablets simvastatin 80 mg are pink colored, capsule shaped, biconvex, film coated tablets, debossed with ‘A’ on one side and ‘04’ on the other side.
4 CONTRAINDICATIONS
- Concomitant administration of strong CYP3A4 inhibitors (e.g., itraconazole, ketoconazole, posaconazole, HIV protease inhibitors, erythromycin, clarithromycin, telithromycin and nefazodone) [see Warnings and Precautions (5.1)].
- Concomitant administration of gemfibrozil, cyclosporine, or danazol [see Warnings and Precautions (5.1)].
- Hypersensitivity to any component of this medication [see Adverse Reactions (6.2)].
- Active liver disease, which may include unexplained persistent elevations in hepatic transaminase levels [see Warnings and Precautions (5.2)].
- Women who are pregnant or may become pregnant. Serum cholesterol and triglycerides increase during normal pregnancy, and cholesterol or cholesterol derivatives are essential for fetal development. Because HMG-CoA reductase inhibitors (statins) decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, simvastatin tablets may cause fetal harm when administered to a pregnant woman. Atherosclerosis is a chronic process and the discontinuation of lipid-lowering drugs during pregnancy should have little impact on the outcome of long-term therapy of primary hypercholesterolemia. There are no adequate and well-controlled studies of use with simvastatin tablets during pregnancy; however, in rare reports congenital anomalies were observed following intrauterine exposure to statins. In rat and rabbit animal reproduction studies, simvastatin revealed no evidence of teratogenicity. Simvastatin tablets should be administered to women of childbearing age only when such patients are highly unlikely to conceive. If the patient becomes pregnant while taking this drug, simvastatin tablets should be discontinued immediately and the patient should be apprised of the potential hazard to the fetus [see Use in Specific Populations (8.1)].
- Nursing mothers. It is not known whether simvastatin is excreted into human milk; however, a small amount of another drug in this class does pass into breast milk. Because statins have the potential for serious adverse reactions in nursing infants, women who require treatment with simvastatin tablets should not breastfeed their infants [see Use in Specific Populations (8.3)].
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy/Rhabdomyolysis
Predisposing
The risk of myopathy, including rhabdomyolysis, is dose related. was
patients
The risk of myopathy, including rhabdomyolysis, is greater in patients on simvastatin 80 mg compared with other statin therapies with similar or greater LDL-C-lowering efficacy and compared with lower doses of simvastatin. Therefore, the 80 mg dose of simvastatin should be used only in patients who have been taking simvastatin 80 mg chronically (e.g., for 12 months or more) without evidence of muscle toxicity [see Dosage and Administration, Restricted Dosing for 80 mg (2.2)]. If, however, a patient who is currently tolerating the 80 mg dose of simvastatin needs to be initiated on an interacting drug that is contraindicated or is associated with a dose cap for simvastatin, that patient should be switched to an alternative statin with less potential for the drug-drug interaction. Patients should be advised of the increased risk of myopathy, including rhabdomyolysis, and to report promptly any unexplained muscle pain, tenderness or weakness. If symptoms occur, treatment should be discontinued immediately. [See Warnings and Precautions (5.2).]
All patients starting therapy with simvastatin, or whose dose of simvastatin is being increased, should be advised of the risk of myopathy, including rhabdomyolysis, and told to report promptly any unexplained muscle pain, tenderness or weakness. Simvastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected. cases
surgical
Drug Interactions
posaconazole[See Contraindications (4) and Drug Interactions (7.1).] In vitro [See Drug Interactions (7.1).]
danazol[see Contraindications (4) and Drug Interactions (7.1 and 7.2)].
agents[see Drug Interactions (7.2)]
and[see Drug Interactions (7.7)]
against[see Drug Interactions (7.3) and Table 3 in Clinical Pharmacology (12.3)]
with[see Drug Interactions (7.4)]
[see also Dosage and Administration (2.3), Drug Interactions (7), Clinical Pharmacology (12.3)]
|
Interacting Agents |
Prescribing Recommendations |
|
Itraconazole Ketoconazole Posaconazole Erythromycin Clarithromycin Telithromycin HIV protease inhibitors Nefazodone Gemfibrozil Cyclosporine Danazol |
Contraindicated with simvastatin |
|
Amiodarone Verapamil Diltiazem |
Do not exceed 10 mg simvastatin daily |
|
Amlodipine Ranolazine |
Do not exceed 20 mg simvastatin daily |
|
Grapefruit juice |
Avoid large quantities of grapefruit juice (>1 quart daily) |
5.2 Liver Dysfunction
Persistent increases (to more than 3X the ULN) in serum transaminases have occurred in approximately 1% of patients who received simvastatin in clinical studies.
[see Clinical Studies (14.1)]
It is recommended that liver function tests be performed before the initiation of treatment, and thereafter when clinically indicated. [see Warnings and Precautions (5.1)]
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Scandinavian Simvastatin Survival Study
| Simvastatin (N = 2,221) % |
Placebo (N = 2,223) % |
|
|
Body as a Whole
Edema/swelling Abdominal pain |
2.7 5.9 |
2.3 5.8 |
|
Cardiovascular System Disorders
Atrial fibrillation |
5.7 |
5.1 |
|
Digestive System Disorders
Constipation Gastritis |
2.2 4.9 |
1.6 3.9 |
|
Endocrine Disorders
Diabetes mellitus |
4.2 |
3.6 |
|
Musculoskeletal Disorders
Myalgia |
3.7 |
3.2 |
|
Nervous System/Psychiatric Disorders
Headache Insomnia Vertigo |
2.5 4 4.5 |
2.1 3.8 4.2 |
|
Respiratory System Disorders
Bronchitis Sinusitis |
6.6 2.3 |
6.3 1.8 |
|
Skin/Skin Appendage Disorders
Eczema |
4.5 |
3 |
|
Urogenital System Disorders
Infection, urinary tract |
3.2 |
3.1 |
Heart Protection Study
Other Clinical Studies
Laboratory Tests
[see Warnings and Precautions (5.2)][See Warnings and Precautions (5.1).]
Adolescent Patients (ages 10 to 17 years)
[see Use in Specific Populations (8.4) and Clinical Studies (14.2)]
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Strong CYP3A4 Inhibitors, Cyclosporine, or Danazol
[See Warnings and Precautions (5.1) and Clinical Pharmacology (12.3).] [see Contraindications (4)].
in vitro [see Warnings and Precautions (5.1)].
[see Contraindications (4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]
7.2 Lipid-Lowering Drugs That Can Cause Myopathy When Given Alone
[see Contraindications (4) and Warnings and Precautions (5.1)]
[see Warnings and Precautions (5.1)]
7.3 Amiodarone, Ranolazine, or Calcium Channel Blockers
[see Dosage and Administration (2.3), Warnings and Precautions (5.1), and Table 3 in Clinical Pharmacology (12.3)]
7.4 Niacin
[See Warnings and Precautions (5.1) and Clinical Pharmacology (12.3).]
7.5 Digoxin
[see Clinical Pharmacology (12.3)]
7.6 Coumarin Anticoagulants
7.7 Colchicine
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category X [See Contraindications (4).]
in utero
2
2
2Reproductive Toxicology
8.3 Nursing Mothers
[see Contraindications (4)]
8.4 Pediatric Use
Doses greater than 40 mg have not been studied in this population. [See Dosage and Administration (2.5), Adverse Reactions (6.1), Clinical Studies (14.2).][see Contraindications (4) and Use in Specific Populations (8.1)].
8.5 Geriatric Use
[See Clinical Pharmacology (12.3).]
[see Clinical Studies (14.1)]
[See Warnings and Precautions (5.1) and Clinical Pharmacology (12.3).]
8.6 Renal Impairment
[See Dosage and Administration (2.6).]
8.7 Hepatic Impairment
[see Contraindications (4) and Warnings and Precautions (5.2)]
10 OVERDOSAGE
22
11 DESCRIPTION
Aspergillus terreus
HSSS25385
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
in vivo
1414
[see Use in Specific Populations (8.5)]
[see Warnings and Precautions (5.1) and Drug Interactions (7.1)]
|
* Results based on a chemical assay except results with propranolol as indicated. † Results could be representative of the following CYP3A4 inhibitors: ketoconazole, erythromycin, clarithromycin, HIV protease inhibitors, and nefazodone. ‡ Simvastatin acid refers to the β-hydroxyacid of simvastatin. § The effect of amounts of grapefruit juice between those used in these two studies on simvastatin pharmacokinetics has not been studied. ¶ Double-strength: one can of frozen concentrate diluted with one can of water. Grapefruit juice was administered TID for 2 days, and 200 mL together with single dose simvastatin and 30 and 90 minutes following single dose simvastatin on Day 3. # Single-strength: one can of frozen concentrate diluted with 3 cans of water. Grapefruit juice was administered with breakfast for 3 days, and simvastatin was administered in the evening on Day 3. Þ Because Chinese patients have an increased risk for myopathy with simvastatin coadministered with lipid-modifying doses (≥ 1 gram/day niacin) of niacin-containing products, and the risk is dose-related, Chinese patients should not receive simvastatin 80 mg coadministered with lipid-modifying doses of niacin-containing products [see Warnings and Precautions (5.1) and Drug Interactions (7.4)]. |
|||||
|
Coadministered Drug or Grapefruit Juice
|
Dosing of Coadministered Drug or Grapefruit Juice
|
Dosing of Simvastatin
|
Geometric Mean Ratio
(Ratio* with/without coadministered drug) No Effect = 1 |
||
|
AUC
|
Cmax
|
||||
|
Contraindicated with simvastatin
[see
Contraindications (4) and
Warnings and Precautions (5.1)]
|
|||||
| Telithromycin†
|
200 mg QD for 4 days |
80 mg |
simvastatin acid‡ simvastatin |
12 8.9 |
15 5.3 |
| Nelfinavir†
|
1250 mg BID for 14 days |
20 mg QD for 28 days |
simvastatin acid‡ simvastatin |
6 |
6.2 |
| Itraconazole†
|
200 mg QD for 4 days |
80 mg |
simvastatin acid‡ simvastatin |
13.1 13.1 |
|
| Posaconazole |
100 mg (oral suspension) QD for 13 days 200 mg (oral suspension) QD for 13 days |
40 mg 40 mg |
simvastatin acid simvastatin simvastatin acid simvastatin |
7.3 10.3 8.5 10.6 |
9.2 9.4 9.5 11.4 |
| Gemfibrozil |
600 mg BID for 3 days |
40 mg |
simvastatin acid simvastatin |
2.85 1.35 |
2.18 0.91 |
|
Avoid >1 quart of grapefruit juice with simvastatin
[see Warnings and Precautions (5.1)]
|
|||||
| Grapefruit Juice§ (high dose) |
200 mL of double-strength TID¶
|
60 mg single dose |
simvastatin acid simvastatin |
7 16 |
|
| Grapefruit Juice§ (low dose) |
8 oz (about 237 mL) of single-strength#
|
20 mg single dose |
simvastatin acid simvastatin |
1.3 1.9 |
|
|
Avoid taking with >10 mg simvastatin, based on clinical and/or postmarketing experience [see Warnings and Precautions (5.1)]
|
|||||
| Verapamil SR |
240 mg QD Days 1 to 7 then 240 mg BID on Days 8 to 10 |
80 mg on Day 10 |
simvastatin acid simvastatin |
2.3 2.5 |
2.4 2.1 |
| Diltiazem |
120 mg BID for 10 days |
80 mg on Day 10 |
simvastatin acid simvastatin |
2.69 3.1 |
2.69 2.88 |
| Diltiazem |
120 mg BID for 14 days |
20 mg on Day 14 |
simvastatin |
4.6 |
3.6 |
| Amiodarone |
400 mg QD for 3 days |
40 mg on Day 3 |
simvastatin acid simvastatin |
1.75 1.76 |
1.72 1.79 |
|
Avoid taking with >20 mg simvastatin, based on clinical and/or postmarketing experience [see Warnings and Precautions (5.1)]
|
|||||
| Amlodipine |
10 mg QD x 10 days |
80 mg on Day 10 |
simvastatin acid simvastatin |
1.58 1.77 |
1.56 1.47 |
| Ranolazine SR |
1000 mg BID for 7 days |
80 mg on Day 1 and Day 6 to 9 |
simvastatin acid simvastatin |
2.26 1.86 |
2.28 1.75 |
|
No dosing adjustments required for the following:
|
|||||
| Fenofibrate |
160 mg QD x 14 days |
80 mg QD on Days 8 to 14 |
simvastatin acid simvastatin |
0.64 0.89 |
0.89 0.83 |
| Niacin extended-releaseÞ
|
2 g single dose |
20 mg single dose |
simvastatin acid simvastatin |
1.6 1.4 |
1.84 1.08 |
| Propranolol |
80 mg single dose |
80 mg single dose |
total inhibitor active inhibitor |
0.79 0.79 |
↓ from 33.6 to 21.1 ng·eq/mL ↓ from 7 to 4.7 ng·eq/mL |
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
in vitroin vitroin vivo
2
13.2 Animal Toxicology and/or Pharmacology
CNS Toxicity
14 CLINICAL STUDIES
14.1 Clinical Studies in Adults
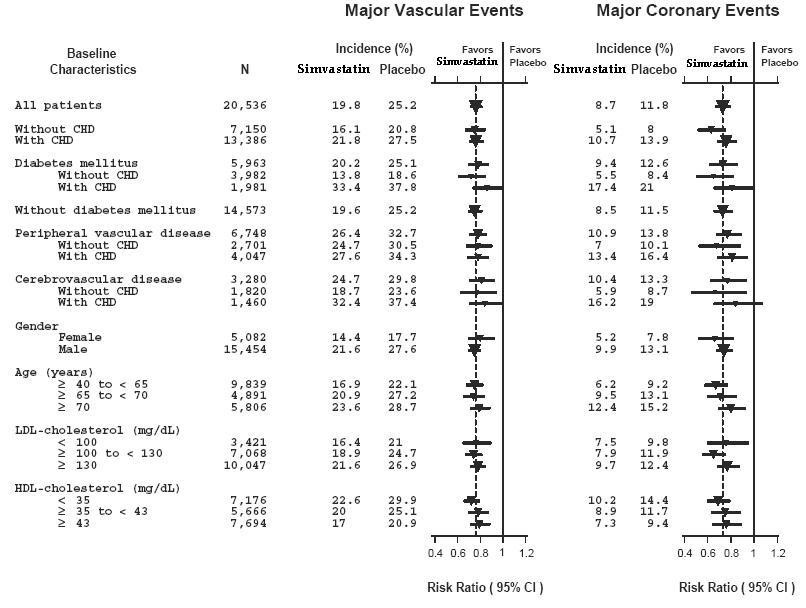
Reductions in Risk of CHD Mortality and Cardiovascular Events
3
3 D.R. Taves, Minimization: a new method of assigning patients to treatment and control groups. Clin. Pharmacol. Ther. 15 (1974), pp. 443-453
|
† n = number of patients with indicated event |
||||
|
Endpoint
|
Simvastatin
(N=10,269) n (%)† |
Placebo
(N=10,267) n (%)† |
Risk Reduction
(%) (95% CI) |
p-Value
|
|
Primary
Mortality CHD mortality |
1,328 (12.9) 587 (5.7) |
1,507 (14.7) 707 (6.9) |
13 (6-19) 18 (8-26) |
p=0.0003 p=0.0005 |
|
Secondary
Non-fatal MI Stroke |
357 (3.5) 444 (4.3) |
574 (5.6) 585 (5.7) |
38 (30-46) 25 (15-34) |
p<0.0001 p<0.0001 |
|
Tertiary
Coronary revascularization Peripheral and other non-coronary revascularization |
513 (5) 450 (4.4) |
725 (7.1) 532 (5.2) |
30 (22-38) 16 (5-26) |
p<0.0001 p=0.006 |
Angiographic Studies
Modifications of Lipid Profiles
Primary Hyperlipidemia (Fredrickson type lla and llb)
|
† median percent change ‡ mean baseline LDL-C 244 mg/dL and median baseline TG 168 mg/dL § mean baseline LDL-C 188 mg/dL and median baseline TG 128 mg/dL || mean baseline LDL-C 226 mg/dL and median baseline TG 156 mg/dL ¶ 21% and 36% median reduction in TG in patients with TG ≤200 mg/dL and TG >200 mg/dL, respectively. Patients with TG >350 mg/dL were excluded †† mean baseline LDL-C 156 mg/dL and median baseline TG 391 mg/dL. |
|||||
| TREATMENT |
N |
TOTAL-C |
LDL-C |
HDL-C |
TG†
|
| Lower Dose Comparative Study‡
(Mean % Change at Week 6) Simvastatin 5 mg q.p.m. Simvastatin 10 mg q.p.m. |
109 110 |
-19 -23 |
-26 -30 |
10 12 |
-12 -15 |
| Scandinavian Simvastatin Survival Study§
(Mean % Change at Week 6) Placebo Simvastatin 20 mg q.p.m. |
2223 2221 |
-1 -28 |
-1 -38 |
0 8 |
-2 -19 |
| Upper Dose Comparative Study||
(Mean % Change Averaged at Weeks 18 and 24) Simvastatin 40 mg q.p.m. Simvastatin 80 mg q.p.m.¶ |
433 664 |
-31 -36 |
-41 -47 |
9 8 |
-18 -24 |
| Multi-Center Combined Hyperlipidemia Study††
(Mean % Change at Week 6) Placebo Simvastatin 40 mg q.p.m Simvastatin 80 mg q.p.m |
125 123 124 |
1 -25 -31 |
2 -29 -36 |
3 13 16 |
-4 -28 -33 |
Hypertriglyceridemia (Fredrickson type IV)
|
† The median baseline values (mg/dL) for the patients in this study were: total-C = 254, LDL-C = 135, HDL-C = 36, TG = 404, VLDL-C = 83, and non-HDL-C = 215. |
|||||||
| TREATMENT |
N |
Total-C |
LDL-C |
HDL-C |
TG |
VLDL-C |
Non-HDL-C |
| Placebo |
74 |
+2 (-7, +7) |
+1 (-8, +14) |
+3 (-3, +10) |
-9 (-25, +13) |
-7 (-25, +11) |
+1 (-9, +8) |
| Simvastatin 40 mg/day |
74 |
-25 (-34, -19) |
-28 (-40, -17) |
+11 (+5, +23) |
-29 (-43, -16) |
-37 (-54, -23) |
-32 (-42, -23) |
| Simvastatin 80 mg/day |
74 |
-32 (-38, -24) |
-37 (-46, -26) |
+15 (+5, +23) |
-34 (-45, -18) |
-41 (-57, -28) |
-38 (-49, -32) |
Dysbetalipoproteinemia (Fredrickson type III)
|
† The median baseline values (mg/dL) were: total-C = 324, LDL-C = 121, HDL-C = 31, TG = 411, VLDL-C = 170, and non-HDL-C = 291. |
|||||||
| TREATMENT |
N |
Total-C |
LDL-C + IDL |
HDL-C |
TG |
VLDL-C + IDL |
Non-HDL-C |
| Placebo |
7 |
-8 (-24, +34) |
-8 (-27, +23) |
-2 (-21, +16) |
+4 (-22, +90) |
-4 (-28, +78) |
-8 (-26, -39) |
| Simvastatin 40 mg/day |
7 |
-50 (-66, -39) |
-50 (-60, -31) |
+7 (-8, +23) |
-41 (-74, -16) |
-58 (-90, -37) |
-57 (-72, -44) |
| Simvastatin 80 mg/day |
7 |
-52 (-55, -41) |
-51 (-57, -28) |
+7 (-5, +29) |
-38 (-58, +2) |
-60 (-72, -39) |
-59 (-61, -46) |
Homozygous Familial Hypercholesterolemia
Endocrine Function
14.2 Clinical Studies in Adolescents
|
† median percent change |
||||||||
| Dosage |
Duration |
N |
Total-C |
LDL-C |
HDL-C |
TG†
|
Apo B |
|
| Placebo |
24 Weeks |
67 |
% Change from Baseline (95% CI) |
1.6 (-2.2, 5.3) |
1.1 (-3.4, 5.5) |
3.6 (-0.7, 8) |
-3.2 (-11.8, 5.4) |
-0.5 (-4.7, 3.6) |
| Mean baseline, mg/dL (SD) |
278.6 (51.8) |
211.9 (49) |
46.9 (11.9) |
90 (50.7) |
186.3 (38.1) |
|||
| Simvastatin |
24 Weeks |
106 |
% Change from Baseline (95% CI) |
-26.5 (-29.6, -23.3) |
-36.8 (-40.5, -33) |
8.3 (4.6, 11.9) |
-7.9 (-15.8, 0) |
-32.4 (-35.9, -29) |
| Mean baseline, mg/dL (SD) |
270.2 (44) |
203.8 (41.5) |
47.7 (9) |
78.3 (46) |
179.9 (33.8) |
|||
16 HOW SUPPLIED/STORAGE AND HANDLING
Simvastatin Tablets USP, 5 mg
Simvastatin Tablets USP, 10 mg
Simvastatin Tablets USP, 20 mg
Simvastatin Tablets USP, 40 mg
Simvastatin Tablets USP, 80 mg
Store at
17 PATIENT COUNSELING INFORMATION
Patients should be advised about substances they should not take concomitantly with simvastatin [see Contraindications (4) and Warnings and Precautions (5.1)]. Patients should also be advised to inform other healthcare professionals prescribing a new medication or increasing the dose of an existing medication that they are taking simvastatin.
17.1 Muscle Pain
Patients using the 80 mg dose should be informed that the risk of myopathy, including rhabdomyolysis, is increased with use of the 80 mg dose.
17.2 Liver Enzymes
17.3 Pregnancy
17.4 Breastfeeding
Aurobindo Pharma USA, Inc.
Aurobindo Pharma Limited
image of label
SimvastatinSimvastatin TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!