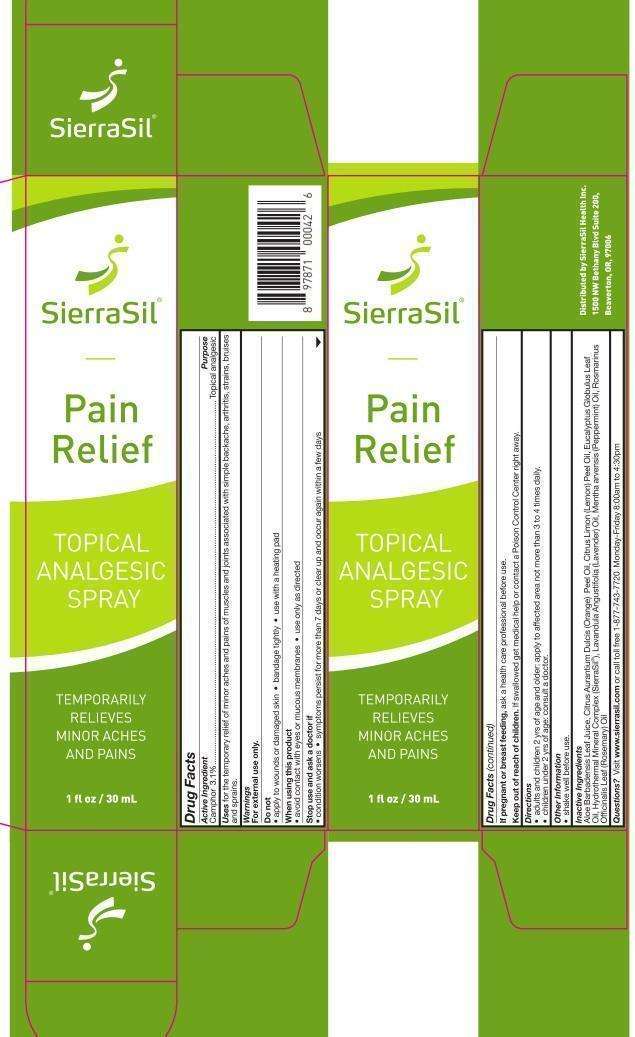
SierraSil Pain Relief
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- SierraSil Pain Relief Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active Ingredient
Camphor 3.1%
Purpose
Camphor 3.1%.........................................Topical analgesic
SierraSil Pain Relief Uses
for the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains, bruises and sprains.
Warnings
For external use only.
Do not
- apply to wounds or damaged skin
- bandage tightly
- use with a heating pad
When using this product
- avoid contact with eyes or mucous membranes
- use only as directed
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
If pregnant or breast feeding, ask a health care professional before use.
Keep out of reach of children.
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- adults and children 2 yrs of age and older: apply to affected area not more than 3 to 4 times daily.
- children under 2 yrs of age: consult a doctor.
Other Information
- shake well before use.
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Citrus Aurantium Dulcis (Orange) Peel Oil, Citrus
Limon (Lemon) Peel Oil, Eucalyptus Globulus Leaf Oil, Hydrothermal Mineral Complex (SierraSil), Lavandula Angustifolia
(Lavender) Oil, Mentha arvensis (Peppermint) Oil, Rosmarinus Officinalis
Leaf (Rosemary) Oil
Questions?
Visit www.sierrasil.com or call toll free 1-877-743-7720 Monday-Friday 8:00am to 4:30pm
Distributed by SierraSil Health Inc.
1500 NW Bethany Blvd Suite 200,
Beaverton, OR, 97006
Principal Display Panel
SierraSil
Pain
Relief
TOPICAL
ANALGESIC
SPRAY
TEMPORARILY
RELIEVES
MINOR ACHES
AND PAINS
1 fl oz / 30 mL
SierraSil Pain ReliefCamphor SPRAY
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||