Sheffield Baby Teething Gel
Faria LLC dba Sheffield Pharmaceuticals
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Sheffield Baby Teething Gel Uses
- Warnings
- Directions
- Sheffield Baby Teething Gel Other information
- Inactive ingredients
- Principal Display Panel – 0.33oz Carton Label
- Principal Display Panel – 0.33oz Tube Label
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Benzocaine 7.5%
Purpose
Oral Teething Pain Reliever
Uses
- for temporary relief of sore gums due to teething in infants and children under 4 months of age and older
Warnings
Allergy alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine or other "caine" anesthetics.
Do not use
- more than directed
- for more than 7 days unless told to do so by a dentist or doctor
When using this product fever and nasal congestion are not symptoms of teething and may indicate the presence of infection. If these symptoms persist, consult a doctor.
Stop use and ask a doctor if
- swelling, rash or fever develops
- irritation, pain, or redness persists or worsens
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- do not use tube if it is cut prior to opening
- cut open tip of tube on score mark
- use your fingertip or cotton applicator to apply a small pea-size amount of teething gel
- apply to affected area up to four times daily or as directed by a dentist or physician
- for infants under 4 months of age, there is no recommended dose or treatment , except under the advice and supervision of a dentist or doctor.
Other information
- store at a controlled room temperature 59°-86°F (15°-30°C)
Inactive ingredients
D&C Yellow No. 10, FD&C Red No. 40, Flavor, Glycerin, PEG 6, PEG 8, PEG 32, PEG 75, Sodium Saccharin, Sorbic Acid, Purified water
Principal Display Panel – 0.33oz Carton Label
NDC 11527-061-47
Baby Teething Gel
Benzocaine 7.5% NET WT. 0.33 OZ (9.35g)
Oral Pain Reliever for Teething
Dr. Sheffield's
SHEAF-FIELD
Made in the USA

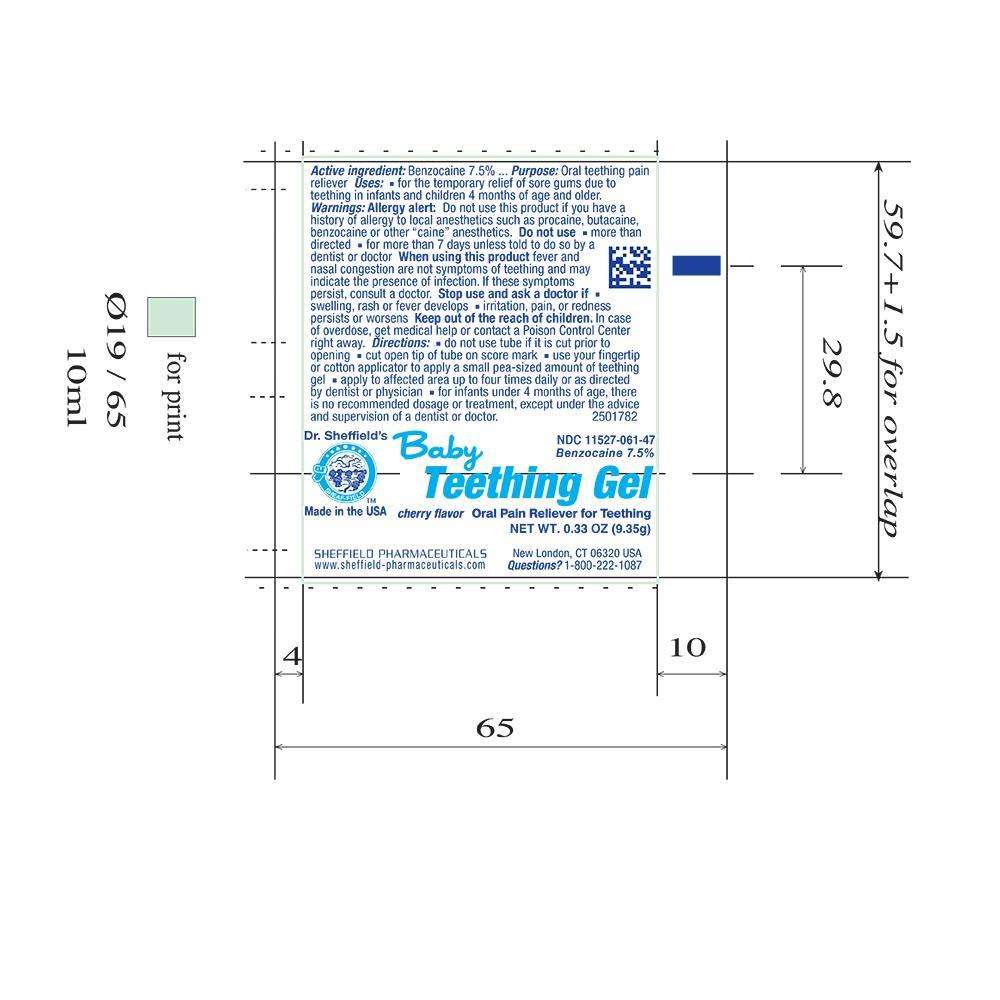
Principal Display Panel – 0.33oz Tube Label
NDC 11527-061-47
Baby Teething Gel
Benzocaine 7.5%
Cherry flavor Oral Pain Reliever for Teething
Dr. Sheffield's
SHEAF-FIELD
Made in the USA
NET WT. 0.33 OZ (9.35g)
Sheffield Baby Teething GelBENZOCAINE GEL
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||