Sertraline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- SERTRALINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- SERTRALINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- SERTRALINE HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Suicidality and Antidepressant DrugsAntidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of Sertraline hydrochloride or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Sertraline hydrochloride tablets are not approved for the treatment of major depressive disorder in pediatric patients. (SeeWARNINGS:Clinical Worsening and Suicide Risk,PRECAUTIONS:Information for Patients, andPRECAUTIONS:Pediatric Use).
SERTRALINE HYDROCHLORIDE DESCRIPTION
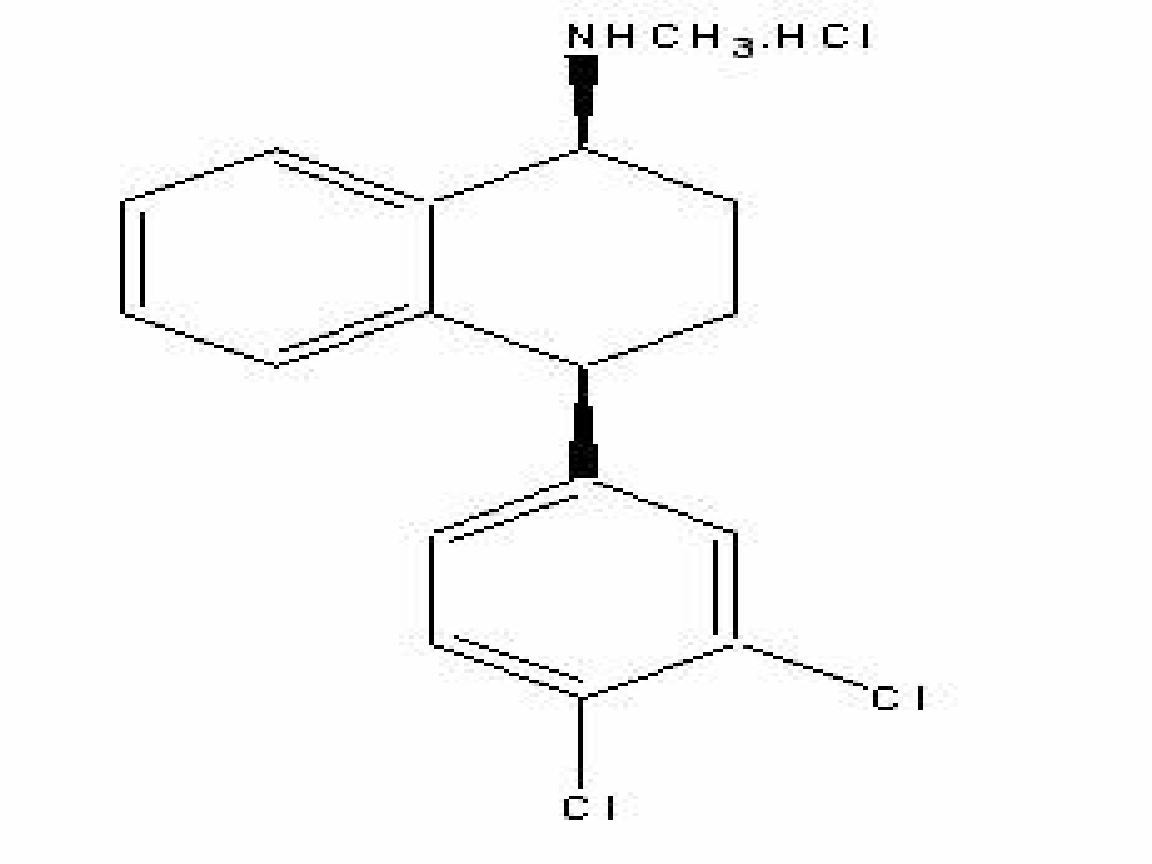
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
Systemic Bioavailability
Metabolism
Protein BindingPRECAUTIONS
Pediatric PharmacokineticsDOSAGE AND ADMINISTRATION
Age
Liver DiseasePRECAUTIONSDOSAGE AND ADMINISTRATION
Renal DiseasePRECAUTIONS
Clinical Trials
Major Depressive Disorder
Premenstrual Dysphoric Disorder (PMDD)
INDICATIONS & USAGE
Major Depressive Disorder
Clinical TrialsCLINICAL PHARMACOLOGY
Clinical TrialsCLINICAL PHARMACOLOGY
Premenstrual Dysphoric Disorder (PMDD)
Clinical TrialsCLINICAL PHARMACOLOGY
DOSAGE AND ADMINISTRATION
SERTRALINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSPRECAUTIONS
WARNINGS
Clinical Worsening and Suicide RiskAll patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
PRECAUTIONSDOSAGE AND ADMINISTRATION
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder:
Cases of serious sometimes-fatal reactions have been reported in patients receiving sertraline hydrochloride a selective serotonin reuptake inhibitor (SSRI), in combination with a monoamine oxidase inhibitor (MAOI). Symptoms of a drug interaction between an SSRI and an MAOI include: hyperthermia, rigidity, myoclonus, autonomic instability with possible rapid fluctuations of vital signs, mental status changes that include confusion, irritability, and extreme agitation progressing to delirium and coma. These reactions have also been reported in patients who have recently discontinued an SSRI and have been started on an MAOI. Some cases presented with features resembling neuroleptic malignant syndrome. Therefore, sertraline hydrochloride should not be used in combination with an MAOI, or within 14 days of discontinuing treatment with an MAOI. Similarly, at least 14 days should be allowed after stopping sertraline hydrochloride before starting an MAOI.
The concomitant use of sertraline hydrochloride with MAOIs intended to treat depression is contraindicated (seeCONTRAINDICATIONSandWARNINGSPotential for Interaction with Monoamine Oxidase Inhibitors.)
Serotonin Syndrome or Neuroleptic Malignant Syndrome (NMS)-like Reactions
PRECAUTIONS
GeneralActivation of Mania/Hypomania
Weight Loss
Seizure
Discontinuation of Treatment with Sertraline hydrochloride
DOSAGE AND ADMINISTRATION
Abnormal Bleeding
Weak Uricosuric Effect
Use in Patients with Concomitant Illness
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGY
Interference with Cognitive and Motor PerformanceInformation for Patients
HyponatremiaGERIATRIC USE
Platelet Function
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk:
LABORATORY TESTS
DRUG INTERACTIONS
Potential Effects of Coadministration of Drugs Highly Bound to Plasma ProteinsCimetidine
CNS Active Drugs
CONTRAINDICATIONS
Monoamine Oxidase InhibitorsCONTRAINDICATIONSWARNINGS
Drugs Metabolized by P450 3A4
Drugs Metabolized by P450 2D6PRECAUTIONS
Serotonergic Drugs:WARNINGSSerotonin SyndromePRECAUTIONSDrug Interactions
Triptans:WARNINGSSerotonin Syndrome
Sumatriptan
Tricyclic Antidepressant Drugs Effective in the Treatment of Major Depressive Disorder (TCAs)PRECAUTIONS
Hypoglycemic Drugs
Atenolol
Digoxin
Microsomal Enzyme Induction
Drugs that Interfere with Hemostasis (Non-selective NSAIDs, Aspirin, Warfarin, etc.)
Electroconvulsive Therapy
Alcohol
Carcinogenesis
Mutagenesis
Impairment of Fertility
PREGNANCY
Pregnancy Category CPregnancy-Nonteratogenic EffectsWARNINGS
DOSAGE AND ADMINISTRATION
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
BOX WARNINGWARNINGSPharmacokineticsCLINICAL PHARMACOLOGY
ADVERSE REACTIONS
The risks, if any, that may be associated with sertraline's use beyond 1 year in children and adolescents have not been systematically assessed. The prescriber should be mindful that the evidence relied upon to conclude that sertraline is safe for use in children and adolescents derives from clinical studies that were 10 to 52 weeks in duration and from the extrapolation of experience gained with adult patients. In particular, there are no studies that directly evaluate the effects of long-term sertraline use on the growth, development, and maturation of children and adolescents. Although there is no affirmative finding to suggest that sertraline possesses a capacity to adversely affect growth, development or maturation, the absence of such findings is not compelling evidence of the absence of the potential of sertraline to have adverse effects in chronic use (seeWARNINGS Clinical Worsening and Suicide Risk).
GERIATRIC USE
ADVERSE REACTIONSPRECAUTIONS, Hyponatremia
SERTRALINE HYDROCHLORIDE ADVERSE REACTIONS
Incidence in Placebo-Controlled Trials
Associated with Discontinuation in Placebo-Controlled Clinical Trials
Male and Female Sexual Dysfunction with SSRIs
Other Adverse Events in Pediatric Patients
Other Events Observed During the Premarketing Evaluation of sertraline hydrochloride
Autonomic Nervous System Disorders
Body as a WholeGeneral Disorders
Cardiovascular
Central and Peripheral Nervous System Disorders
Disorders of Skin and Appendages
Endocrine Disorders
Gastrointestinal Disorders
General
Hearing and Vestibular Disorders
Hematopoietic and Lymphatic
Liver and Biliary System Disorders
Metabolic and Nutritional Disorders
Musculoskeletal System Disorders
Psychiatric Disorders
Reproductive
Respiratory System Disorders
Special Senses
Urinary System Disorders
Laboratory Tests
Other Events Observed During the Postmarketing Evaluation of Sertraline hydrochloride
DRUG ABUSE AND DEPENDENCE
Controlled Substance ClassPhysical and Psychological Dependence
OVERDOSAGE
Human ExperienceOverdose Management
DOSAGE & ADMINISTRATION
Initial TreatmentDosage for Adults
Major Depressive Disorder
Premenstrual Dysphoric Disorder
Clinical TrialsCLINICAL PHARMACOLOGY
Maintenance/Continuation/Extended Treatment
Major Depressive DisorderClinical TrialsCLINICAL PHARMACOLOGY
Premenstrual Dysphoric Disorder
Switching Patients to or from a Monoamine Oxidase InhibitorCONTRAINDICATIONSWARNINGS
Special Populations
Dosage for Hepatically Impaired PatientsCLINICAL PHARMACOLOGYPRECAUTIONS
Treatment of Pregnant Women During the Third TrimesterPRECAUTIONS
Discontinuation of Treatment with sertraline hydrochloride
PRECAUTIONS
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Antidepressant Medicines, Depression and other Serious Mental Illnesses, and Suicidal Thoughts or ActionsTalk to your, or your family member's, healthcare provider about:
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and talking (mania)
-
● other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
-
● Never stop an antidepressant medicine without first talking to a healthcare provider.Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses.It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects.Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines.Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children.Talk to your child's healthcare provider for more information.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Sertraline HydrochlorideSertraline Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!